Nanoemulsions and nanocapsules as carriers for the development of intranasal mRNA vaccines
- PMID: 38811465
- PMCID: PMC11208213
- DOI: 10.1007/s13346-024-01635-5
Nanoemulsions and nanocapsules as carriers for the development of intranasal mRNA vaccines
Abstract
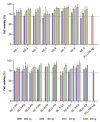
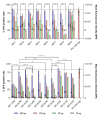
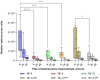
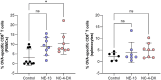
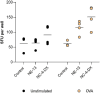
The global emergency of coronavirus disease 2019 (COVID-19) has spurred extensive worldwide efforts to develop vaccines for protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Our contribution to this global endeavor involved the development of a diverse library of nanocarriers, as alternatives to lipid nanoparticles (LNPs), including nanoemulsions (NEs) and nanocapsules (NCs), with the aim of protecting and delivering messenger ribonucleic acid (mRNA) for nasal vaccination purposes. A wide range of prototypes underwent rigorous screening through a series of in vitro and in vivo experiments, encompassing assessments of cellular transfection, cytotoxicity, and intramuscular administration of a model mRNA for protein translation. As a result, two promising candidates were identified for nasal administration. One of them was a NE incorporating a combination of an ionizable lipid (C12-200) and cationic lipid (DOTAP), both intended to condense mRNA, along with DOPE, which is known to facilitate endosomal escape. This NE exhibited a size of 120 nm and a highly positive surface charge (+ 50 mV). Another candidate was an NC formulation comprising the same components and endowed with a dextran sulfate shell. This formulation showed a size of 130 nm and a moderate negative surface charge (-16 mV). Upon intranasal administration of mRNA encoding for ovalbumin (mOVA) associated with optimized versions of the said NE and NCs, a robust antigen-specific CD8 + T cell response was observed. These findings underscore the potential of NEs and polymeric NCs in advancing mRNA vaccine development for combating infectious diseases.
Keywords: Intranasal vaccination; Nanoparticles; Polymeric nanocapsule; SARS-CoV-2; mRNA vaccine.
© 2024. The Author(s).
Conflict of interest statement
M.J.A. is a founder and shareholder of Libera Bio. M.L.B. is an employee of Eli Lilly & Company, Spain. G.L. is an employee of Anjarium Biosciences, Switzerland. S.A. is an employee of Sanofi Pasteur, France.
Figures
References
-
- World Health Organization, WHO Coronavirus (COVID-19) Dashboard. (2023). https://covid19.who.int/ (accessed May 29, 2023).
-
- Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT, Dinnon KH, Elbashir SM, Shaw CA, Woods A, Fritch EJ, Martinez DR, Bock KW, Minai M, Nagata BM, Hutchinson GB, Wu K, Henry C, Bahl K, Garcia-Dominguez D, Ma LZ, Renzi I, Kong WP, Schmidt SD, Wang L, Zhang Y, Phung E, Chang LA, Loomis RJ, Altaras NE, Narayanan E, Metkar M, Presnyak V, Liu C, Louder MK, Shi W, Leung K, Yang ES, West A, Gully KL, Stevens LJ, Wang N, Wrapp D, Doria-Rose NA, Stewart-Jones G, Bennett H, Alvarado GS, Nason MC, Ruckwardt TJ, McLellan JS, Denison MR, Chappell JD, Moore IN, Morabito KM, Mascola JR, Baric RS, Carfi A, Graham BS. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–71. doi: 10.1038/s41586-020-2622-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

