Narrative Review of Brivaracetam: Preclinical Profile and Clinical Benefits in the Treatment of Patients with Epilepsy
- PMID: 38811492
- PMCID: PMC11213745
- DOI: 10.1007/s12325-024-02876-z
Narrative Review of Brivaracetam: Preclinical Profile and Clinical Benefits in the Treatment of Patients with Epilepsy
Abstract
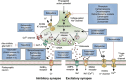
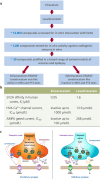
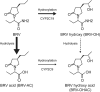
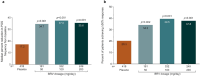
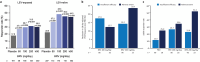
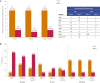
One third of patients with epilepsy will continue to have uncontrolled seizures despite treatment with antiseizure medications (ASMs). There is therefore a need to develop novel ASMs. Brivaracetam (BRV) is an ASM that was developed in a major drug discovery program aimed at identifying selective, high-affinity synaptic vesicle protein 2A (SV2A) ligands, the target molecule of levetiracetam. BRV binds to SV2A with 15- to 30-fold higher affinity and greater selectivity than levetiracetam. BRV has broad-spectrum antiseizure activity in animal models of epilepsy, a favorable pharmacokinetic profile, few clinically relevant drug-drug interactions, and rapid brain penetration. BRV is available in oral and intravenous formulations and can be initiated at target dose without titration. Efficacy and safety of adjunctive BRV (50-200 mg/day) treatment of focal-onset seizures was demonstrated in three pivotal phase III trials (NCT00490035/NCT00464269/NCT01261325), including in patients who had previously failed levetiracetam. Efficacy and safety of adjunctive BRV were also demonstrated in adult Asian patients with focal-onset seizures (NCT03083665). In several open-label trials (NCT00150800/NCT00175916/NCT01339559), long-term safety and tolerability of adjunctive BRV was established, with efficacy maintained for up to 14 years, with high retention rates. Evidence from daily clinical practice highlights BRV effectiveness and tolerability in specific epilepsy patient populations with high unmet needs: the elderly (≥ 65 years of age), children (< 16 years of age), patients with cognitive impairment, patients with psychiatric comorbid conditions, and patients with acquired epilepsy of specific etiologies (post-stroke epilepsy/brain tumor related epilepsy/traumatic brain injury-related epilepsy). Here, we review the preclinical profile and clinical benefits of BRV from pivotal trials and recently published evidence from daily clinical practice.
Keywords: Antiseizure medication; Comorbidities; Drug-drug interactions; Effectiveness; Elderly; Fast response; Focal-onset seizures; Real-world evidence; SV2A; Tolerability.
Plain language summary
One in three people with epilepsy continue to have seizures despite treatment. Brivaracetam is a medicine used to treat seizures in people with epilepsy. It binds to a protein in the brain (synaptic vesicle protein 2A) and is effective in many different animal models of epilepsy. Brivaracetam enters the brain quickly. It has few interactions with other medicines, which is important because people with epilepsy may be taking additional medicines for epilepsy or other conditions. Brivaracetam is available as tablets, oral solution, and solution for intravenous injection, can be started at the recommended target dose, and is easy to use. In three phase III trials, people with uncontrolled focal-onset seizures taking brivaracetam 50–200 mg each day had fewer seizures than people taking a placebo. Brivaracetam was tolerated well. It also worked well in many people who had previously not responded to antiseizure medications. The efficacy of brivaracetam treatment is maintained for up to 14 years. Brivaracetam treatment reduces seizures in the elderly (≥ 65 years old), in children (< 16 years old), in people with cognitive or learning disabilities, in people with additional psychiatric conditions, and in people with different causes of epilepsy (post-stroke epilepsy, brain-tumor related epilepsy, and traumatic brain injury-related epilepsy). Here, we review brivaracetam characteristics and the results when people with epilepsy received brivaracetam in key clinical trials and real-world studies in daily clinical practice.
© 2024. The Author(s).
Conflict of interest statement
Pavel Klein has served as a consultant, advisory board member or speaker for Abbott, Angelini, Aquestive, Arvelle Therapeutics, Aucta Pharmaceuticals, Dr. Reddy’s, Eisai, Jazz Pharmaceuticals, Neurelis, Neurona, SK Life Science, Sunovion, UCB Pharma, UNEEG, UniQure; is a member of the Medical Advisory Board of Stratus and of the Scientific Advisory Board of OB Pharma; is the CEO of PrevEp, Inc; and has received research support from CURE/Department of Defense and from the NIH/SBIR. Dimitrios Bourikas is a salaried employee of UCB Pharma and has received UCB Pharma stock from his employment.
Figures
Similar articles
-
Efficacy, safety, and tolerability of adjunctive brivaracetam in adult Asian patients with uncontrolled focal-onset seizures: A phase III randomized, double-blind, placebo-controlled trial.Epilepsia Open. 2024 Jun;9(3):1007-1020. doi: 10.1002/epi4.12929. Epub 2024 Apr 4. Epilepsia Open. 2024. PMID: 38576178 Free PMC article. Clinical Trial.
-
Retention, efficacy, tolerability, and quality of life during long-term adjunctive brivaracetam treatment by number of lifetime antiseizure medications: A post hoc analysis of phase 3 trials in adults with focal seizures.Epilepsy Behav. 2023 Jan;138:108967. doi: 10.1016/j.yebeh.2022.108967. Epub 2022 Nov 23. Epilepsy Behav. 2023. PMID: 36435010 Clinical Trial.
-
Efficacy, safety, and tolerability of adjunctive brivaracetam for secondarily generalized tonic-clonic seizures: Pooled results from three Phase III studies.Epilepsy Res. 2016 Nov;127:179-185. doi: 10.1016/j.eplepsyres.2016.09.003. Epub 2016 Sep 3. Epilepsy Res. 2016. PMID: 27608437 Clinical Trial.
-
Brivaracetam: Rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment.Epilepsia. 2016 Apr;57(4):538-48. doi: 10.1111/epi.13340. Epub 2016 Feb 26. Epilepsia. 2016. PMID: 26920914 Review.
-
Brivaracetam for the treatment of focal-onset seizures: pharmacokinetic and pharmacodynamic evaluations.Expert Opin Drug Metab Toxicol. 2020 Oct;16(10):853-863. doi: 10.1080/17425255.2020.1813277. Epub 2020 Sep 20. Expert Opin Drug Metab Toxicol. 2020. PMID: 32853036
Cited by
-
Antiseizure Medications: Advancements, Challenges, and Prospects in Drug Development.Curr Neuropharmacol. 2025;23(8):879-906. doi: 10.2174/011570159X323666241029171256. Curr Neuropharmacol. 2025. PMID: 39865817 Free PMC article. Review.
References
-
- National Institute for Health and Care Excellence. Epilepsies in children, young people and adults 2022. www.nice.org.uk/guidance/ng217. Accessed 27 Feb 2024. - PubMed
-
- Gunasekera CL, Sirven JI, Feyissa AM. The evolution of antiseizure medication therapy selection in adults: is artificial intelligence -assisted antiseizure medication selection ready for prime time? J Cent Nerv Syst Dis. 2023;15:11795735231209209. doi: 10.1177/11795735231209209. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

