Targeting BCL9/BCL9L enhances antigen presentation by promoting conventional type 1 dendritic cell (cDC1) activation and tumor infiltration
- PMID: 38811552
- PMCID: PMC11137111
- DOI: 10.1038/s41392-024-01838-9
Targeting BCL9/BCL9L enhances antigen presentation by promoting conventional type 1 dendritic cell (cDC1) activation and tumor infiltration
Abstract
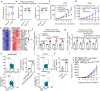
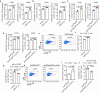
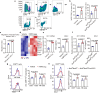
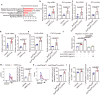
Conventional type 1 dendritic cells (cDC1) are the essential antigen-presenting DC subset in antitumor immunity. Suppressing B-cell lymphoma 9 and B-cell lymphoma 9-like (BCL9/BCL9L) inhibits tumor growth and boosts immune responses against cancer. However, whether oncogenic BCL9/BCL9L impairs antigen presentation in tumors is still not completely understood. Here, we show that targeting BCL9/BCL9L enhanced antigen presentation by stimulating cDC1 activation and infiltration into tumor. Pharmacological inhibition of BCL9/BCL9L with a novel inhibitor hsBCL9z96 or Bcl9/Bcl9l knockout mice markedly delayed tumor growth and promoted antitumor CD8+ T cell responses. Mechanistically, targeting BCL9/BCL9L promoted antigen presentation in tumors. This is due to the increase of cDC1 activation and tumor infiltration by the XCL1-XCR1 axis. Importantly, using single-cell transcriptomics analysis, we found that Bcl9/Bcl9l deficient cDC1 were superior to wild-type (WT) cDC1 at activation and antigen presentation via NF-κB/IRF1 signaling. Together, we demonstrate that targeting BCL9/BCL9L plays a crucial role in cDC1-modulated antigen presentation of tumor-derived antigens, as well as CD8+ T cell activation and tumor infiltration. Targeting BCL9/BCL9L to regulate cDC1 function and directly orchestrate a positive feedback loop necessary for optimal antitumor immunity could serve as a potential strategy to counter immune suppression and enhance cancer immunotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Extracellular vesicles derived from mature dendritic cells loaded with cDC1-specific chemokine XCL1 combined with chemotherapy-induced ICD for the treatment of castration-resistant prostate cancer.Cancer Immunol Immunother. 2025 Jun 18;74(8):242. doi: 10.1007/s00262-025-04070-8. Cancer Immunol Immunother. 2025. PMID: 40531239 Free PMC article.
-
Intratumoral delivery of a highly active form of XCL1 enhances antitumor CTL responses through recruitment of CXCL9-expressing conventional type-1 dendritic cells.Int J Cancer. 2024 Jun 15;154(12):2176-2188. doi: 10.1002/ijc.34874. Epub 2024 Feb 12. Int J Cancer. 2024. PMID: 38346928
-
cDC1s Promote Atherosclerosis via Local Immunity and Are Targetable for Therapy.Circ Res. 2025 Jul 18;137(3):400-416. doi: 10.1161/CIRCRESAHA.124.325792. Epub 2025 May 30. Circ Res. 2025. PMID: 40444360 Free PMC article.
-
Rewriting the dendritic cell code in cancer-from subset identity to immunotherapeutic design.FEBS Lett. 2025 Jul;599(14):2060-2083. doi: 10.1002/1873-3468.70108. Epub 2025 Jul 16. FEBS Lett. 2025. PMID: 40667699 Free PMC article. Review.
-
Oncolytic immunovirotherapy: finding the tumor antigen needle in the antiviral haystack.Immunotherapy. 2025 Jun;17(8):585-594. doi: 10.1080/1750743X.2025.2513853. Epub 2025 Jun 6. Immunotherapy. 2025. PMID: 40474818 Review.
Cited by
-
CXCL6 Reshapes Lipid Metabolism and Induces Neutrophil Extracellular Trap Formation in Cholangiocarcinoma Progression and Immunotherapy Resistance.Adv Sci (Weinh). 2025 Jul;12(27):e2503009. doi: 10.1002/advs.202503009. Epub 2025 Apr 30. Adv Sci (Weinh). 2025. PMID: 40305734 Free PMC article.
-
Multi-Omics Analysis Identifies Immune Regulatory Networks in Sepsis-Associated Liver Injury: Experimental Validation and Clinical Relevance.J Inflamm Res. 2025 Aug 9;18:10711-10722. doi: 10.2147/JIR.S515615. eCollection 2025. J Inflamm Res. 2025. PMID: 40809463 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

