G protein-specific mechanisms in the serotonin 5-HT2A receptor regulate psychosis-related effects and memory deficits
- PMID: 38811567
- PMCID: PMC11137019
- DOI: 10.1038/s41467-024-48196-2
G protein-specific mechanisms in the serotonin 5-HT2A receptor regulate psychosis-related effects and memory deficits
Abstract
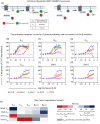
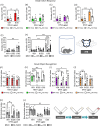
G protein-coupled receptors (GPCRs) are sophisticated signaling machines able to simultaneously elicit multiple intracellular signaling pathways upon activation. Complete (in)activation of all pathways can be counterproductive for specific therapeutic applications. This is the case for the serotonin 2 A receptor (5-HT2AR), a prominent target for the treatment of schizophrenia. In this study, we elucidate the complex 5-HT2AR coupling signature in response to different signaling probes, and its physiological consequences by combining computational modeling, in vitro and in vivo experiments with human postmortem brain studies. We show how chemical modification of the endogenous agonist serotonin dramatically impacts the G protein coupling profile of the 5-HT2AR and the associated behavioral responses. Importantly, among these responses, we demonstrate that memory deficits are regulated by Gαq protein activation, whereas psychosis-related behavior is modulated through Gαi1 stimulation. These findings emphasize the complexity of GPCR pharmacology and physiology and open the path to designing improved therapeutics for the treatment of stchizophrenia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

