Mucus production, host-microbiome interactions, hormone sensitivity, and innate immune responses modeled in human cervix chips
- PMID: 38811586
- PMCID: PMC11137093
- DOI: 10.1038/s41467-024-48910-0
Mucus production, host-microbiome interactions, hormone sensitivity, and innate immune responses modeled in human cervix chips
Abstract
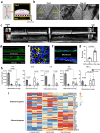
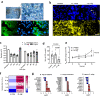
Modulation of the cervix by steroid hormones and commensal microbiome play a central role in the health of the female reproductive tract. Here we describe organ-on-a-chip (Organ Chip) models that recreate the human cervical epithelial-stromal interface with a functional epithelial barrier and production of mucus with biochemical and hormone-responsive properties similar to living cervix. When Cervix Chips are populated with optimal healthy versus dysbiotic microbial communities (dominated by Lactobacillus crispatus and Gardnerella vaginalis, respectively), significant differences in tissue innate immune responses, barrier function, cell viability, proteome, and mucus composition are observed that are similar to those seen in vivo. Thus, human Cervix Organ Chips represent physiologically relevant in vitro models to study cervix physiology and host-microbiome interactions, and hence may be used as a preclinical testbed for development of therapeutic interventions to enhance women's health.
© 2024. The Author(s).
Conflict of interest statement
D.E.I. holds equity in Emulate, chairs its scientific advisory board and is a member of its board of directors. The remaining authors declare no competing interests.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

