Development of a novel non-invasive biomarker panel for hepatic fibrosis in MASLD
- PMID: 38811591
- PMCID: PMC11137090
- DOI: 10.1038/s41467-024-48956-0
Development of a novel non-invasive biomarker panel for hepatic fibrosis in MASLD
Abstract
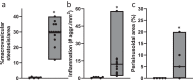
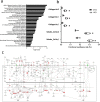
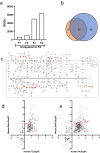
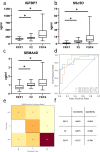
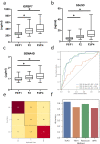
Accurate non-invasive biomarkers to diagnose metabolic dysfunction-associated steatotic liver disease (MASLD)-related fibrosis are urgently needed. This study applies a translational approach to develop a blood-based biomarker panel for fibrosis detection in MASLD. A molecular gene expression signature identified from a diet-induced MASLD mouse model (LDLr-/-.Leiden) is translated into human blood-based biomarkers based on liver biopsy transcriptomic profiles and protein levels in MASLD patient serum samples. The resulting biomarker panel consists of IGFBP7, SSc5D and Sema4D. LightGBM modeling using this panel demonstrates high accuracy in predicting MASLD fibrosis stage (F0/F1: AUC = 0.82; F2: AUC = 0.89; F3/F4: AUC = 0.87), which is replicated in an independent validation cohort. The overall accuracy of the model outperforms predictions by the existing markers Fib-4, APRI and FibroScan. In conclusion, here we show a disease mechanism-related blood-based biomarker panel with three biomarkers which is able to identify MASLD patients with mild or advanced hepatic fibrosis with high accuracy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
TNO has a patent filed for the use of protein biomarkers for NAFLD (Inventors: L.V., A.v.K., and R.H.). Other authors declare no competing interests.
Figures


References
-
- Rinella, M. E. et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 78, 1966-1986 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

