Advanced magnetic resonance imaging detects altered placental development in pregnancies affected by congenital heart disease
- PMID: 38811636
- PMCID: PMC11136986
- DOI: 10.1038/s41598-024-63087-8
Advanced magnetic resonance imaging detects altered placental development in pregnancies affected by congenital heart disease
Abstract
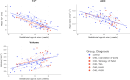
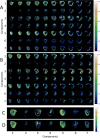
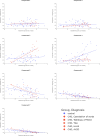
Congenital heart disease (CHD) is the most common congenital malformation and is associated with adverse neurodevelopmental outcomes. The placenta is crucial for healthy fetal development and placental development is altered in pregnancy when the fetus has CHD. This study utilized advanced combined diffusion-relaxation MRI and a data-driven analysis technique to test the hypothesis that placental microstructure and perfusion are altered in CHD-affected pregnancies. 48 participants (36 controls, 12 CHD) underwent 67 MRI scans (50 control, 17 CHD). Significant differences in the weighting of two independent placental and uterine-wall tissue components were identified between the CHD and control groups (both pFDR < 0.001), with changes most evident after 30 weeks gestation. A significant trend over gestation in weighting for a third independent tissue component was also observed in the CHD cohort (R = 0.50, pFDR = 0.04), but not in controls. These findings add to existing evidence that placental development is altered in CHD. The results may reflect alterations in placental perfusion or the changes in fetal-placental flow, villous structure and maturation that occur in CHD. Further research is needed to validate and better understand these findings and to understand the relationship between placental development, CHD, and its neurodevelopmental implications.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Advanced magnetic resonance imaging detects altered placental development in pregnancies affected by congenital heart disease.Res Sq [Preprint]. 2024 Jan 23:rs.3.rs-3873412. doi: 10.21203/rs.3.rs-3873412/v1. Res Sq. 2024. Update in: Sci Rep. 2024 May 29;14(1):12357. doi: 10.1038/s41598-024-63087-8. PMID: 38343847 Free PMC article. Updated. Preprint.
Similar articles
-
Advanced magnetic resonance imaging detects altered placental development in pregnancies affected by congenital heart disease.Res Sq [Preprint]. 2024 Jan 23:rs.3.rs-3873412. doi: 10.21203/rs.3.rs-3873412/v1. Res Sq. 2024. Update in: Sci Rep. 2024 May 29;14(1):12357. doi: 10.1038/s41598-024-63087-8. PMID: 38343847 Free PMC article. Updated. Preprint.
-
Non-Invasive Placental Perfusion Imaging in Pregnancies Complicated by Fetal Heart Disease Using Velocity-Selective Arterial Spin Labeled MRI.Sci Rep. 2017 Nov 23;7(1):16126. doi: 10.1038/s41598-017-16461-8. Sci Rep. 2017. PMID: 29170468 Free PMC article.
-
T2* placental MRI in pregnancies complicated with fetal congenital heart disease.Placenta. 2021 May;108:23-31. doi: 10.1016/j.placenta.2021.02.015. Epub 2021 Mar 9. Placenta. 2021. PMID: 33798991 Free PMC article.
-
Placenta morphology and biomarkers in pregnancies with congenital heart disease - A systematic review.Placenta. 2021 Sep 1;112:189-196. doi: 10.1016/j.placenta.2021.07.297. Epub 2021 Aug 8. Placenta. 2021. PMID: 34388551
-
Neuroplacentology in congenital heart disease: placental connections to neurodevelopmental outcomes.Pediatr Res. 2022 Mar;91(4):787-794. doi: 10.1038/s41390-021-01521-7. Epub 2021 Apr 16. Pediatr Res. 2022. PMID: 33864014 Free PMC article. Review.
Cited by
-
Quantitative and longitudinal assessment of human placental inflammation using diffusion basis spectrum imaging.NPJ Womens Health. 2025;3(1):1. doi: 10.1038/s44294-024-00049-5. Epub 2025 Jan 3. NPJ Womens Health. 2025. PMID: 39759173 Free PMC article.
-
Foetal cortical expansion is associated with neurodevelopmental outcome at 2-years in congenital heart disease: a longitudinal follow-up study.EBioMedicine. 2025 Apr;114:105679. doi: 10.1016/j.ebiom.2025.105679. Epub 2025 Mar 29. EBioMedicine. 2025. PMID: 40158387 Free PMC article.
-
Genetic and Environmental Contributors To Congenital Heart Disease.Curr Treat Options Cardiovasc Med. 2025;27(1):36. doi: 10.1007/s11936-025-01091-5. Epub 2025 May 26. Curr Treat Options Cardiovasc Med. 2025. PMID: 40438121 Free PMC article. Review.
-
Structural Covariance Networks in the Fetal Brain Reveal Altered Neurodevelopment for Specific Subtypes of Congenital Heart Disease.J Am Heart Assoc. 2024 Nov 5;13(21):e035880. doi: 10.1161/JAHA.124.035880. Epub 2024 Oct 25. J Am Heart Assoc. 2024. PMID: 39450739 Free PMC article.
References
-
- Heazell, A. The placenta and adverse pregnancy outcomes—opening the black box? BMC Pregn. Childbirth15(Suppl 1), (2015).
-
- EUROCAT. European Platform on Rare Disease Registration (2020).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

