The complete sequence and comparative analysis of ape sex chromosomes
- PMID: 38811727
- PMCID: PMC11168930
- DOI: 10.1038/s41586-024-07473-2
The complete sequence and comparative analysis of ape sex chromosomes
Abstract
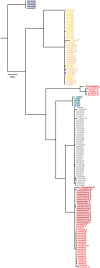
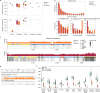
Apes possess two sex chromosomes-the male-specific Y chromosome and the X chromosome, which is present in both males and females. The Y chromosome is crucial for male reproduction, with deletions being linked to infertility1. The X chromosome is vital for reproduction and cognition2. Variation in mating patterns and brain function among apes suggests corresponding differences in their sex chromosomes. However, owing to their repetitive nature and incomplete reference assemblies, ape sex chromosomes have been challenging to study. Here, using the methodology developed for the telomere-to-telomere (T2T) human genome, we produced gapless assemblies of the X and Y chromosomes for five great apes (bonobo (Pan paniscus), chimpanzee (Pan troglodytes), western lowland gorilla (Gorilla gorilla gorilla), Bornean orangutan (Pongo pygmaeus) and Sumatran orangutan (Pongo abelii)) and a lesser ape (the siamang gibbon (Symphalangus syndactylus)), and untangled the intricacies of their evolution. Compared with the X chromosomes, the ape Y chromosomes vary greatly in size and have low alignability and high levels of structural rearrangements-owing to the accumulation of lineage-specific ampliconic regions, palindromes, transposable elements and satellites. Many Y chromosome genes expand in multi-copy families and some evolve under purifying selection. Thus, the Y chromosome exhibits dynamic evolution, whereas the X chromosome is more stable. Mapping short-read sequencing data to these assemblies revealed diversity and selection patterns on sex chromosomes of more than 100 individual great apes. These reference assemblies are expected to inform human evolution and conservation genetics of non-human apes, all of which are endangered species.
© 2024. The Author(s).
Conflict of interest statement
E.E.E. is a member of the scientific advisory board of Variant Bio. R.J.O. is a member of the scientific advisory board of Colossal Biosciences. C.L. is a member of the scientific advisory boards of Nabsys and Genome Insight. The other authors declare no competing interests.
Figures
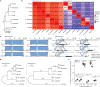

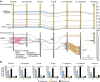
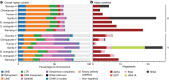






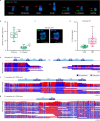

Update of
-
The Complete Sequence and Comparative Analysis of Ape Sex Chromosomes.bioRxiv [Preprint]. 2023 Dec 1:2023.11.30.569198. doi: 10.1101/2023.11.30.569198. bioRxiv. 2023. Update in: Nature. 2024 Jun;630(8016):401-411. doi: 10.1038/s41586-024-07473-2. PMID: 38077089 Free PMC article. Updated. Preprint.
References
-
- Fan, Y. & Silber, S. J. in GeneReviews (eds Adam, M. P. et al.) (Univ. of Washington, Seattle, 2002).
Publication types
MeSH terms
Grants and funding
- R35 GM124827/GM/NIGMS NIH HHS/United States
- R01 GM130691/GM/NIGMS NIH HHS/United States
- U41 HG007497/HG/NHGRI NIH HHS/United States
- R35 GM142916/GM/NIGMS NIH HHS/United States
- R01 CA266339/CA/NCI NIH HHS/United States
- R01 GM146462/GM/NIGMS NIH HHS/United States
- R01 HG010169/HG/NHGRI NIH HHS/United States
- U01 CA253481/CA/NCI NIH HHS/United States
- R01 HG011274/HG/NHGRI NIH HHS/United States
- R35 GM146886/GM/NIGMS NIH HHS/United States
- R01 GM123312/GM/NIGMS NIH HHS/United States
- U24 HG007497/HG/NHGRI NIH HHS/United States
- R01 HG002385/HG/NHGRI NIH HHS/United States
- T32 GM102057/GM/NIGMS NIH HHS/United States
- R01 HG011641/HG/NHGRI NIH HHS/United States
- R35 GM133747/GM/NIGMS NIH HHS/United States
- U24 HG010263/HG/NHGRI NIH HHS/United States
- R01 GM136684/GM/NIGMS NIH HHS/United States
- R35 GM146926/GM/NIGMS NIH HHS/United States
- R35 GM151945/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

