Molecular basis for differential Igk versus Igh V(D)J joining mechanisms
- PMID: 38811728
- PMCID: PMC11153149
- DOI: 10.1038/s41586-024-07477-y
Molecular basis for differential Igk versus Igh V(D)J joining mechanisms
Abstract
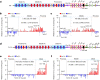
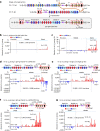
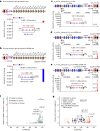
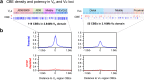
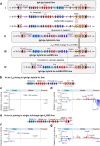
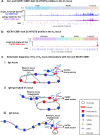
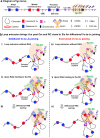
In developing B cells, V(D)J recombination assembles exons encoding IgH and Igκ variable regions from hundreds of gene segments clustered across Igh and Igk loci. V, D and J gene segments are flanked by conserved recombination signal sequences (RSSs) that target RAG endonuclease1. RAG orchestrates Igh V(D)J recombination upon capturing a JH-RSS within the JH-RSS-based recombination centre1-3 (RC). JH-RSS orientation programmes RAG to scan upstream D- and VH-containing chromatin that is presented in a linear manner by cohesin-mediated loop extrusion4-7. During Igh scanning, RAG robustly utilizes only D-RSSs or VH-RSSs in convergent (deletional) orientation with JH-RSSs4-7. However, for Vκ-to-Jκ joining, RAG utilizes Vκ-RSSs from deletional- and inversional-oriented clusters8, inconsistent with linear scanning2. Here we characterize the Vκ-to-Jκ joining mechanism. Igk undergoes robust primary and secondary rearrangements9,10, which confounds scanning assays. We therefore engineered cells to undergo only primary Vκ-to-Jκ rearrangements and found that RAG scanning from the primary Jκ-RC terminates just 8 kb upstream within the CTCF-site-based Sis element11. Whereas Sis and the Jκ-RC barely interacted with the Vκ locus, the CTCF-site-based Cer element12 4 kb upstream of Sis interacted with various loop extrusion impediments across the locus. Similar to VH locus inversion7, DJH inversion abrogated VH-to-DJH joining; yet Vκ locus or Jκ inversion allowed robust Vκ-to-Jκ joining. Together, these experiments implicated loop extrusion in bringing Vκ segments near Cer for short-range diffusion-mediated capture by RC-based RAG. To identify key mechanistic elements for diffusional V(D)J recombination in Igk versus Igh, we assayed Vκ-to-JH and D-to-Jκ rearrangements in hybrid Igh-Igk loci generated by targeted chromosomal translocations, and pinpointed remarkably strong Vκ and Jκ RSSs. Indeed, RSS replacements in hybrid or normal Igk and Igh loci confirmed the ability of Igk-RSSs to promote robust diffusional joining compared with Igh-RSSs. We propose that Igk evolved strong RSSs to mediate diffusional Vκ-to-Jκ joining, whereas Igh evolved weaker RSSs requisite for modulating VH joining by RAG-scanning impediments.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Comment in
-
RAGging on recombination signal sequence strength for diffusion-mediated recombination.Immunol Cell Biol. 2024 Sep;102(8):648-650. doi: 10.1111/imcb.12803. Epub 2024 Jul 7. Immunol Cell Biol. 2024. PMID: 38973226
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

