Vimentin is a key regulator of cell mechanosensing through opposite actions on actomyosin and microtubule networks
- PMID: 38811770
- PMCID: PMC11137025
- DOI: 10.1038/s42003-024-06366-4
Vimentin is a key regulator of cell mechanosensing through opposite actions on actomyosin and microtubule networks
Abstract
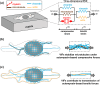
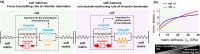
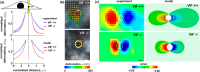
The cytoskeleton is a complex network of interconnected biopolymers consisting of actin filaments, microtubules, and intermediate filaments. These biopolymers work in concert to transmit cell-generated forces to the extracellular matrix required for cell motility, wound healing, and tissue maintenance. While we know cell-generated forces are driven by actomyosin contractility and balanced by microtubule network resistance, the effect of intermediate filaments on cellular forces is unclear. Using a combination of theoretical modeling and experiments, we show that vimentin intermediate filaments tune cell stress by assisting in both actomyosin-based force transmission and reinforcement of microtubule networks under compression. We show that the competition between these two opposing effects of vimentin is regulated by the microenvironment stiffness. These results reconcile seemingly contradictory results in the literature and provide a unified description of vimentin's effects on the transmission of cell contractile forces to the extracellular matrix.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA261694/CA/NCI NIH HHS/United States
- CMMI-154857/National Science Foundation (NSF)
- R01GM136259/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- MCB-1750462/National Science Foundation (NSF)
- R01 EB017753/EB/NIBIB NIH HHS/United States
- MRSEC/DMR-1720530/National Science Foundation (NSF)
- R01 GM155943/GM/NIGMS NIH HHS/United States
- R01 EB030876/EB/NIBIB NIH HHS/United States
- R01GM127621/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- DMS-1953572/National Science Foundation (NSF)
- R35GM142963/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources

