PRSS3/mesotrypsin as a putative regulator of the biophysical characteristics of epidermal keratinocytes in superficial layers
- PMID: 38811772
- PMCID: PMC11137022
- DOI: 10.1038/s41598-024-63271-w
PRSS3/mesotrypsin as a putative regulator of the biophysical characteristics of epidermal keratinocytes in superficial layers
Abstract
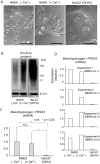
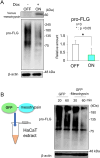
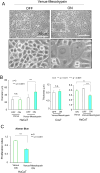
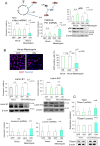
Mesotrypsin, encoded by the PRSS3 gene, is a distinctive trypsin isoform renowned for its exceptional resistance to traditional trypsin inhibitors and unique substrate specificity. Within the skin epidermis, this protein primarily expresses in the upper layers of the stratified epidermis and plays a crucial role in processing pro-filaggrin (Pro-FLG). Although prior studies have partially elucidated its functions using primary cultured keratinocytes, challenges persist due to these cells' differentiation-activated cell death program. In the present study, HaCaT keratinocytes, characterized by minimal endogenous mesotrypsin expression and sustained proliferation in differentiated states, were utilized to further scrutinize the function of mesotrypsin. Despite the ready degradation of the intact form of active mesotrypsin in these cells, fusion with Venus, flanked by a peptide linker, enables evasion from the protein elimination machinery, thus facilitating activation of the Pro-FLG processing system. Inducing Venus-mesotrypsin expression in the cells resulted in a flattened phenotype and reduced proliferative capacity. Moreover, these cells displayed altered F-actin assembly, enhanced E-cadherin adhesive activity, and facilitated tight junction formation without overtly influencing epidermal differentiation. These findings underscore mesotrypsin's potentially pivotal role in shaping the characteristic cellular morphology of upper epidermal layers.
Keywords: Cell shape; Epidermis; Growth arrest; Keratinocyte; PRSS3/mesotrypsin; Tight junction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Keratinocyte-specific mesotrypsin contributes to the desquamation process via kallikrein activation and LEKTI degradation.J Invest Dermatol. 2014 Jun;134(6):1665-1674. doi: 10.1038/jid.2014.3. Epub 2014 Jan 3. J Invest Dermatol. 2014. PMID: 24390132
-
PRSS3/mesotrypsin is a therapeutic target for metastatic prostate cancer.Mol Cancer Res. 2012 Dec;10(12):1555-66. doi: 10.1158/1541-7786.MCR-12-0314. Mol Cancer Res. 2012. PMID: 23258495 Free PMC article.
-
Keratinocytes synthesize enteropeptidase and multiple forms of trypsinogen during terminal differentiation.J Invest Dermatol. 2010 Apr;130(4):944-52. doi: 10.1038/jid.2009.364. Epub 2009 Nov 19. J Invest Dermatol. 2010. PMID: 19924134
-
Exonic mutations associated with atopic dermatitis disrupt lympho-epithelial Kazal-type related inhibitor action and enhance its degradation.Allergy. 2020 Feb;75(2):403-411. doi: 10.1111/all.14018. Epub 2019 Sep 9. Allergy. 2020. PMID: 31407378
-
Mesotrypsin and caspase-14 participate in prosaposin processing: potential relevance to epidermal permeability barrier formation.J Biol Chem. 2014 Jul 18;289(29):20026-38. doi: 10.1074/jbc.M113.543421. Epub 2014 May 28. J Biol Chem. 2014. PMID: 24872419 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

