A novel small-molecule PCSK9 inhibitor E28362 ameliorates hyperlipidemia and atherosclerosis
- PMID: 38811775
- PMCID: PMC11420243
- DOI: 10.1038/s41401-024-01305-9
A novel small-molecule PCSK9 inhibitor E28362 ameliorates hyperlipidemia and atherosclerosis
Abstract
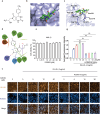
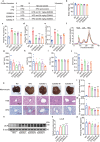
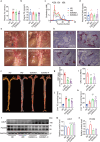
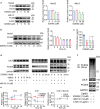
Proprotein convertase subtilisin/kexin type 9 (PCSK9) binds to the epidermal growth factor precursor homologous domain A (EGF-A) of low-density lipoprotein receptor (LDLR) in the liver and triggers the degradation of LDLR via the lysosomal pathway, consequently leading to an elevation in plasma LDL-C levels. Inhibiting PCSK9 prolongs the lifespan of LDLR and maintains cholesterol homeostasis in the body. Thus, PCSK9 is an innovative pharmacological target for treating hypercholesterolemia and atherosclerosis. In this study, we discovered that E28362 was a novel small-molecule PCSK9 inhibitor by conducting a virtual screening of a library containing 40,000 compounds. E28362 (5, 10, 20 μM) dose-dependently increased the protein levels of LDLR in both total protein and the membrane fraction in both HepG2 and AML12 cells, and enhanced the uptake of DiI-LDL in AML12 cells. MTT assay showed that E28362 up to 80 μM had no obvious toxicity in HepG2, AML12, and HEK293a cells. The effects of E28362 on hyperlipidemia and atherosclerosis were evaluated in three different animal models. In high-fat diet-fed golden hamsters, administration of E28362 (6.7, 20, 60 mg·kg-1·d-1, i.g.) for 4 weeks significantly reduced plasma total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C) and PCSK9 levels, and reduced liver TC and TG contents. In Western diet-fed ApoE-/- mice (20, 60 mg·kg-1·d-1, i.g.) and human PCSK9 D374Y overexpression mice (60 mg·kg-1·d-1, i.g.), administration of E28362 for 12 weeks significantly decreased plasma LDL-C levels and the area of atherosclerotic lesions in en face aortas and aortic roots. Moreover, E28362 significantly increased the protein expression level of LDLR in the liver. We revealed that E28362 selectively bound to PCSK9 in HepG2 and AML12 cells, blocked the interaction between LDLR and PCSK9, and induced the degradation of PCSK9 through the ubiquitin-proteasome pathway, which finally resulted in increased LDLR protein levels. In conclusion, E28362 can block the interaction between PCSK9 and LDLR, induce the degradation of PCSK9, increase LDLR protein levels, and alleviate hyperlipidemia and atherosclerosis in three distinct animal models, suggesting that E28362 is a promising lead compound for the treatment of hyperlipidemia and atherosclerosis.
Keywords: E28362; LDLR; PCSK9 inhibitor; atherosclerosis; hyperlipidemia.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. - PubMed
-
- Arvanitis M, Lowenstein CJ. Dyslipidemia. Ann Intern Med. 2023;176:ITC81–ITC96. - PubMed
-
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–46. - PubMed
-
- Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: a consensus statement from the european atherosclerosis society consensus panel. Eur Heart J. 2020;41:2313–30. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

