Nebulised interferon beta-1a (SNG001) in the treatment of viral exacerbations of COPD
- PMID: 38811970
- PMCID: PMC11138078
- DOI: 10.1186/s12931-024-02854-7
Nebulised interferon beta-1a (SNG001) in the treatment of viral exacerbations of COPD
Abstract
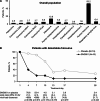
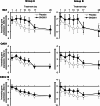
Background: Respiratory viral infections are major drivers of chronic obstructive pulmonary disease (COPD) exacerbations. Interferon-β is naturally produced in response to viral infection, limiting replication. This exploratory study aimed to demonstrate proof-of-mechanism, and evaluate the efficacy and safety of inhaled recombinant interferon-β1a (SNG001) in COPD. Part 1 assessed the effects of SNG001 on induced sputum antiviral interferon-stimulated gene expression, sputum differential cell count, and respiratory function. Part 2 compared SNG001 and placebo on clinical efficacy, sputum and serum biomarkers, and viral clearance.
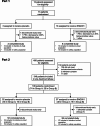
Methods: In Part 1, patients (N = 13) with stable COPD were randomised 4:1 to SNG001 or placebo once-daily for three days. In Part 2, patients (N = 109) with worsening symptoms and a positive respiratory viral test were randomised 1:1 to SNG001 or placebo once-daily for 14 days in two Groups: A (no moderate exacerbation); B (moderate COPD exacerbation [i.e., acute worsening of respiratory symptoms treated with antibiotics and/or oral corticosteroids]).
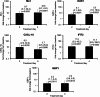
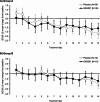
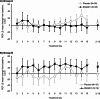
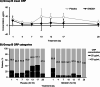
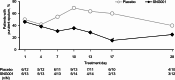
Results: In Part 1, SNG001 upregulated sputum interferon gene expression. In Part 2, there were minimal SNG001-placebo differences in the efficacy endpoints; however, whereas gene expression was initially upregulated by viral infection, then declined on placebo, levels were maintained with SNG001. Furthermore, the proportion of patients with detectable rhinovirus (the most common virus) on Day 7 was lower with SNG001. In Group B, serum C-reactive protein and the proportion of patients with purulent sputum increased with placebo (suggesting bacterial infection), but not with SNG001. The overall adverse event incidence was similar with both treatments.
Conclusions: Overall, SNG001 was well-tolerated in patients with COPD, and upregulated lung antiviral defences to accelerate viral clearance. These findings warrant further investigation in a larger study.
Trial registration: EU clinical trials register (2017-003679-75), 6 October 2017.
Keywords: Biomarkers; Chronic obstructive pulmonary disease; Interferons; Symptom flare up.
© 2024. The Author(s).
Conflict of interest statement
In addition to writing support, the authors have the following conflicts of interest to declare.
PDM is an employee of Synairgen Research plc, the parent company of Synairgen Research Ltd (and such costs are met by Synairgen Research Ltd), the sponsor of this trial, and owns shares and has options on shares in Synairgen plc.
JLB is an employee of Synairgen Research Ltd, the sponsor of this trial, and has options on shares in Synairgen plc.
VJT is an employee of Synairgen Research Ltd, the sponsor of this trial, and owns shares and has options on shares in Synairgen plc.
TNB provided statistical support, programming and consultancy to Synairgen Research Ltd via a contract with his employer, Veramed Ltd.
CN has no other conflicts of interest to disclose.
MM provided consulting services to Synairgen Research Ltd, the sponsor of this trial, with all payments made to tranScrip Ltd.
MGC declares grants and funding to his institution from AstraZeneca for investigator-sponsored and AstraZeneca-sponsored studies, to his institution from Phillips Research for collaborative research, and to him and his institution from the National Institute for Health and Care Research, consulting fees from Synairgen PLC, AstraZeneca, and Chiesi, honoraria for lectures/educational meetings from AstraZeneca, Chiesi, Boehringer Ingelheim, GlaxoSmithKline, and Pfizer, and support to attend congresses from AstraZeneca and Chiesi, all outside the scope of this manuscript.
DS declares the receipt of consulting fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Synairgen, Teva, Theravance, and Verona, all outside the scope of this manuscript.
RC declares an AstraZeneca grant for an investigator-led study within a Medical Research Council project, payment or honoraria for lectures from GlaxoSmithKline, AstraZeneca, Teva, Chiesi, Sanofi, and Novartis, support for attending conferences from Chiesi, Sanofi, and GlaxoSmithKline, and participation in advisory boards for GlaxoSmithKline, AstraZeneca, and Celltrion, all outside the scope of this manuscript.
BL reports investigator fees and fees for the conduct of the current study. Outside the scope of this manuscript, he has no other conflicts of interest to disclose.
KL is an employee of Synairgen Research Ltd, the sponsor of this trial, and has options on shares in Synairgen plc.
SR is an employee of Synairgen Research Ltd, the sponsor of this trial, and has options on shares in Synairgen plc.
SD is an employee of Synairgen Research Ltd, the sponsor of this trial, and owns shares and has options on shares in Synairgen plc.
FJG declares the receipt of consulting fees paid to tranScrip Ltd from Synairgen Research plc, the sponsor of this trial, and participation in a Data Safety Monitoring Board for Synairgen. She is also president of the Faculty of Pharmaceutical Medicine of three UK Royal College of Physicians.
STH received payments as non-executive director of, and owns shares in, Synairgen plc, the parent company of the sponsor of this trial.
RD declares the receipt of consulting fees and payment for participation in a Data Safety Monitoring Board or Advisory Board from Synairgen Research Ltd, the sponsor of this trial. RD owns shares in Synairgen plc, the parent company of the sponsor of this trial. Outside the trial, he declares payment or honoraria from Regeneron, GlaxoSmithKline and Kymab.
TMAW received research funding and consultancy fees from Synairgen Research Ltd, the sponsor of this trial. Outside the trial, he declares research grants from the National Institute for Health and Care Research, Medical Research Council, Bergenbio, AstraZeneca, UCB and Janssen, consultancy fees from AstraZeneca, Valneva, Olam Pharma, Janssen and My mHealth, lecture fees from AstraZeneca, Boehringer Ingelheim and Roche, participation on a Data Safety Monitoring Board for Valneva, and that he holds stock in My mHealth.
Figures
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet]. 2024 [cited 2024 Mar 18]. https://goldcopd.org/2024-gold-report/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

