Motor imagery-based brain-computer interface rehabilitation programs enhance upper extremity performance and cortical activation in stroke patients
- PMID: 38812014
- PMCID: PMC11134735
- DOI: 10.1186/s12984-024-01387-w
Motor imagery-based brain-computer interface rehabilitation programs enhance upper extremity performance and cortical activation in stroke patients
Abstract
Background: The most challenging aspect of rehabilitation is the repurposing of residual functional plasticity in stroke patients. To achieve this, numerous plasticity-based clinical rehabilitation programs have been developed. This study aimed to investigate the effects of motor imagery (MI)-based brain-computer interface (BCI) rehabilitation programs on upper extremity hand function in patients with chronic hemiplegia.
Design: A 2010 Consolidated Standards for Test Reports (CONSORT)-compliant randomized controlled trial.
Methods: Forty-six eligible stroke patients with upper limb motor dysfunction participated in the study, six of whom dropped out. The patients were randomly divided into a BCI group and a control group. The BCI group received BCI therapy and conventional rehabilitation therapy, while the control group received conventional rehabilitation only. The Fugl-Meyer Assessment of the Upper Extremity (FMA-UE) score was used as the primary outcome to evaluate upper extremity motor function. Additionally, functional magnetic resonance imaging (fMRI) scans were performed on all patients before and after treatment, in both the resting and task states. We measured the amplitude of low-frequency fluctuation (ALFF), regional homogeneity (ReHo), z conversion of ALFF (zALFF), and z conversion of ReHo (ReHo) in the resting state. The task state was divided into four tasks: left-hand grasping, right-hand grasping, imagining left-hand grasping, and imagining right-hand grasping. Finally, meaningful differences were assessed using correlation analysis of the clinical assessments and functional measures.
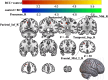
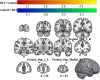
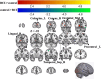
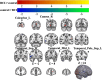
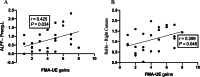
Results: A total of 40 patients completed the study, 20 in the BCI group and 20 in the control group. Task-related blood-oxygen-level-dependent (BOLD) analysis showed that when performing the motor grasping task with the affected hand, the BCI group exhibited significant activation in the ipsilateral middle cingulate gyrus, precuneus, inferior parietal gyrus, postcentral gyrus, middle frontal gyrus, superior temporal gyrus, and contralateral middle cingulate gyrus. When imagining a grasping task with the affected hand, the BCI group exhibited greater activation in the ipsilateral superior frontal gyrus (medial) and middle frontal gyrus after treatment. However, the activation of the contralateral superior frontal gyrus decreased in the BCI group relative to the control group. Resting-state fMRI revealed increased zALFF in multiple cerebral regions, including the contralateral precentral gyrus and calcarine and the ipsilateral middle occipital gyrus and cuneus, and decreased zALFF in the ipsilateral superior temporal gyrus in the BCI group relative to the control group. Increased zReHo in the ipsilateral cuneus and contralateral calcarine and decreased zReHo in the contralateral middle temporal gyrus, temporal pole, and superior temporal gyrus were observed post-intervention. According to the subsequent correlation analysis, the increase in the FMA-UE score showed a positive correlation with the mean zALFF of the contralateral precentral gyrus (r = 0.425, P < 0.05), the mean zReHo of the right cuneus (r = 0.399, P < 0.05).
Conclusion: In conclusion, BCI therapy is effective and safe for arm rehabilitation after severe poststroke hemiparesis. The correlation of the zALFF of the contralateral precentral gyrus and the zReHo of the ipsilateral cuneus with motor improvements suggested that these values can be used as prognostic measures for BCI-based stroke rehabilitation. We found that motor function was related to visual and spatial processing, suggesting potential avenues for refining treatment strategies for stroke patients.
Trial registration: The trial is registered in the Chinese Clinical Trial Registry (number ChiCTR2000034848, registered July 21, 2020).
Keywords: Brain–computer interface (BCI); Fugl–Meyer Assessment of the Upper Extremity (FMA-UE); Motor imagery (MI); Stroke rehabilitation; fMRI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Jiang Y, Yin J, Zhao B, Zhang Y, Peng T, Zhuang W, et al. Motor imagery brain–computer interface in rehabilitation of upper limb motor dysfunction after stroke. J Vis Exp. 2023. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

