Characterization of novel extracellular proteases produced by Acanthamoeba castellanii after contact with human corneal epithelial cells and their relevance to pathogenesis
- PMID: 38812022
- PMCID: PMC11137893
- DOI: 10.1186/s13071-024-06304-7
Characterization of novel extracellular proteases produced by Acanthamoeba castellanii after contact with human corneal epithelial cells and their relevance to pathogenesis
Abstract
Background: Proteases produced by Acanthamoeba spp. play an important role in their virulence and may be the key to understanding Acanthamoeba pathogenesis; thus, increasing attention has been directed towards these proteins. The present study aimed to investigate the lytic factors produced by Acanthamoeba castellanii during the first hours of in vitro co-culture with human corneal epithelial cells (HCECs).
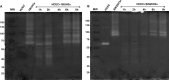
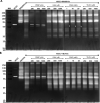
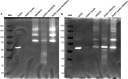
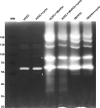
Methods: We used one old and one recent Acanthamoeba isolate, both from patients with severe keratitis, and subsets of these strains with enhanced pathogenic potential induced by sequential passaging over HCEC monolayers. The proteolytic profiles of all strains and substrains were examined using 1D in-gel zymography.
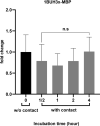
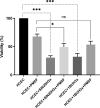
Results: We observed the activity of additional proteases (ranging from 33 to 50 kDa) during the early interaction phase between amoebae and HCECs, which were only expressed for a short time. Based on their susceptibilities to protease inhibitors, these proteases were characterized as serine proteases. Protease activities showed a sharp decline after 4 h of co-incubation. Interestingly, the expression of Acanthamoeba mannose-binding protein did not differ between amoebae in monoculture and those in co-culture. Moreover, we observed the activation of matrix metalloproteinases in HCECs after contact with Acanthamoeba.
Conclusions: This study revealed the involvement of two novel serine proteases in Acanthamoeba pathogenesis and suggests a pivotal role of serine proteases during Acanthamoeba-host cell interaction, contributing to cell adhesion and lysis.
Keywords: Acanthamoeba; Host‐pathogen interaction; Human corneal epithelial cells; Mannose-binding protein; Metalloproteinase; Pathogenesis; Protease inhibitor; Serine protease; Virulence factors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing of interest with the contents of this article.
Figures



Similar articles
-
Extracellular proteases of Acanthamoeba castellanii (encephalitis isolate belonging to T1 genotype) contribute to increased permeability in an in vitro model of the human blood-brain barrier.J Infect. 2005 Aug;51(2):150-6. doi: 10.1016/j.jinf.2004.09.001. J Infect. 2005. PMID: 16038767
-
Comparison of specific activity and cytopathic effects of purified 33 kDa serine proteinase from Acanthamoeba strains with different degree of virulence.Korean J Parasitol. 2006 Dec;44(4):321-30. doi: 10.3347/kjp.2006.44.4.321. Korean J Parasitol. 2006. PMID: 17170574 Free PMC article.
-
Mannose induces the release of cytopathic factors from Acanthamoeba castellanii.Infect Immun. 1998 Jan;66(1):5-10. doi: 10.1128/IAI.66.1.5-10.1998. Infect Immun. 1998. PMID: 9423832 Free PMC article.
-
The pathogenesis of Acanthamoeba keratitis.Microbes Infect. 1999 May;1(6):437-43. doi: 10.1016/s1286-4579(99)80047-1. Microbes Infect. 1999. PMID: 10602676 Review.
-
Proteases of Acanthamoeba.Parasitol Res. 2023 Dec 8;123(1):19. doi: 10.1007/s00436-023-08059-z. Parasitol Res. 2023. PMID: 38063887 Review.
Cited by
-
Targets for the diagnosis of Acanthamoeba eye infections include four cyst wall proteins and the mannose-binding domain of the trophozoite mannose-binding protein.mSphere. 2025 Mar 25;10(3):e0094824. doi: 10.1128/msphere.00948-24. Epub 2025 Mar 4. mSphere. 2025. PMID: 40035521 Free PMC article.
-
The pathogenesis, risk factors, diagnosis and treatment of Acanthamoeba keratitis.Front Med (Lausanne). 2025 Jul 24;12:1559224. doi: 10.3389/fmed.2025.1559224. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40776920 Free PMC article. Review.
References
-
- Randag AC, van Rooij J, van Goor AT, Verkerk S, Wisse RPL, Saelens IEY, Stoutenbeek R, van Dooren BTH, Cheng YYY, Eggink CA. The rising incidence of Acanthamoeba keratitis: a 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS ONE. 2019;14:e0222092. doi: 10.1371/journal.pone.0222092. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

