Condensation methodology for quantification of Polymyxin B fluorimetrically: application to pharmaceutical formulations and greenness assessment
- PMID: 38812036
- PMCID: PMC11137906
- DOI: 10.1186/s13065-024-01156-9
Condensation methodology for quantification of Polymyxin B fluorimetrically: application to pharmaceutical formulations and greenness assessment
Abstract
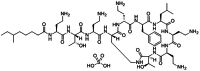
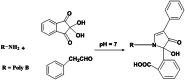
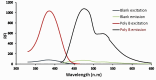
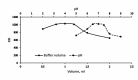
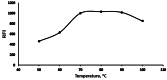
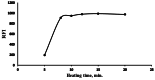
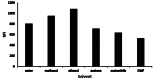
The appearance of multidrug-resistant Gram-negative bacterial infections, along with the lack of newly discovered antibiotics, resulted in the return to old antimicrobial medications like Polymyxins. As a result, the suggested technique aims to develop a fast, environmentally friendly, and sensitive fluorimetric method for quantifying Polymyxin B. The investigated approach depends on generating a highly fluorescent derivative by a condensation pathway between the studied drug and ninhydrin in the presence of phenylacetaldehyde and then estimated spectrofluorimetrically. After the reaction conditions were well optimized, the fluorescent product was estimated at emission wavelength (λem) = 475.5 nm (following excitation at a wavelength (λex) = 386 nm. The developed calibration plot displayed rectilinear throughout the following range (0.2-3 µg mL- 1), and the calculated limit of detection and quantification were 0.062 µg mL- 1 and 0.187 µg mL- 1, respectively. As a consequence, the drug's ophthalmic and intravenous pharmaceutical forms were both successfully quantified with an excellent degree of recovery. Finally, the methodology's greenness was assessed utilizing Analytical Eco-Scale scores.
Keywords: Methodology’s greenness; Ninhydrin; Ophthalmic and intravenous pharmaceutical forms; Phenylacetaldehyde; Polymyxin B.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Diaryl pyrrolone fluorescent probing strategy for Mirabegron determination through condensation with ninhydrin and phenylacetaldehyde: Application to dosage forms, human urine and plasma.Spectrochim Acta A Mol Biomol Spectrosc. 2024 Oct 5;318:124515. doi: 10.1016/j.saa.2024.124515. Epub 2024 May 23. Spectrochim Acta A Mol Biomol Spectrosc. 2024. PMID: 38810435
-
Salvage parenteral antibiotics for multidrug-resistant (MDR) Gram-negative bacteria; a fluorescamine-based technique for ultrasensitive spectrofluorimetric measurement of Polymyxins; human plasma application.Luminescence. 2022 Jun;37(6):971-979. doi: 10.1002/bio.4245. Epub 2022 Apr 18. Luminescence. 2022. PMID: 35393741
-
New validated spectrofluorimetric protocol for colistin assay through condensation with 2,2-dihydroxyindan-1,3-dione: application to content uniformity testing.RSC Adv. 2022 Nov 23;12(52):33559-33566. doi: 10.1039/d2ra04259b. eCollection 2022 Nov 22. RSC Adv. 2022. PMID: 36505680 Free PMC article.
-
Clinical use of intravenous polymyxin B for the treatment of patients with multidrug-resistant Gram-negative bacterial infections: An evaluation of the current evidence.J Glob Antimicrob Resist. 2021 Mar;24:342-359. doi: 10.1016/j.jgar.2020.12.026. Epub 2021 Jan 21. J Glob Antimicrob Resist. 2021. PMID: 33486122 Review.
-
Polymyxins-Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment.Antioxidants (Basel). 2020 Jun 9;9(6):506. doi: 10.3390/antiox9060506. Antioxidants (Basel). 2020. PMID: 32526966 Free PMC article. Review.
Cited by
-
Full green assay of parenteral dosage forms of polymyxins utilizing xanthene dye: application to content uniformity testing.BMC Chem. 2024 Aug 27;18(1):158. doi: 10.1186/s13065-024-01261-9. BMC Chem. 2024. PMID: 39192355 Free PMC article.
-
Spectrofluorimetric determination of tapinarof via Zn (II) complexation and assessment of its topical dosage application.BMC Chem. 2024 Sep 10;18(1):165. doi: 10.1186/s13065-024-01271-7. BMC Chem. 2024. PMID: 39252088 Free PMC article.
References
-
- Setiawati H, Harmita H, Suryadi H. Development and validation method for simultaneous quantification of neomycin and polymyxin B by HPLC-ELSD and comparison with microbiological method. J Appl Pharm Sci. 2020 doi: 10.7324/JAPS.2020.104016. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
