Tissue-specific sex differences in pediatric and adult immune cell composition and function
- PMID: 38812520
- PMCID: PMC11133680
- DOI: 10.3389/fimmu.2024.1373537
Tissue-specific sex differences in pediatric and adult immune cell composition and function
Abstract
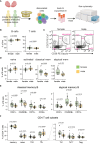
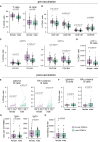
Sex-based differences in immune cell composition and function can contribute to distinct adaptive immune responses. Prior work has quantified these differences in peripheral blood, but little is known about sex differences within human lymphoid tissues. Here, we characterized the composition and phenotypes of adaptive immune cells from male and female ex vivo tonsils and evaluated their responses to influenza antigens using an immune organoid approach. In a pediatric cohort, female tonsils had more memory B cells compared to male tonsils direct ex vivo and after stimulation with live-attenuated but not inactivated vaccine, produced higher influenza-specific antibody responses. Sex biases were also observed in adult tonsils but were different from those measured in children. Analysis of peripheral blood immune cells from in vivo vaccinated adults also showed higher frequencies of tissue homing CD4 T cells in female participants. Together, our data demonstrate that distinct memory B and T cell profiles are present in male vs. female lymphoid tissues and peripheral blood respectively and suggest that these differences may in part explain sex biases in response to vaccines and viruses.
Keywords: adaptive immunity; influenza vaccine; pediatric immunity; sex differences; tonsil organoids.
Copyright © 2024 Mitul, Kastenschmidt, Sureshchandra, Wagoner, Sorn, Mcllwain, Hernandez-Davies, Jain, de Assis, Trask, Davies and Wagar.
Conflict of interest statement
LW is a co-inventor on a patent, owned by Stanford University, that describes the immune organoid culture technique. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

