4,4-Dimethylsterols Reduces Fat Accumulation via Inhibiting Fatty Acid Amide Hydrolase In Vitro and In Vivo
- PMID: 38812531
- PMCID: PMC11134202
- DOI: 10.34133/research.0377
4,4-Dimethylsterols Reduces Fat Accumulation via Inhibiting Fatty Acid Amide Hydrolase In Vitro and In Vivo
Abstract
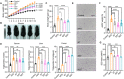
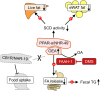
4,4-Dimethylsterols constitute a unique class of phytosterols responsible for regulating endogenous cannabinoid system (ECS) functions. However, precise mechanism through which 4,4-dimethylsterols affect fat metabolism and the linkage to the ECS remain unresolved. In this study, we identified that 4,4-dimethylsterols, distinct from 4-demethseterols, act as inhibitors of fatty acid amide hydrolases (FAAHs) both in vivo and in vitro. Genetic ablation of FAAHs (faah-1) abolishes the effects of 4,4-dimethylsterols on fat accumulation and locomotion behavior in a Caenorhabditis elegans model. We confirmed that dietary intervention with 4,4-dimethylsterols in a high-fat diet (HFD) mouse model leads to a significant reduction in body weight (>11.28%) with improved lipid profiles in the liver and adipose tissues and increased fecal triacylglycerol excretion. Untargeted and targeted metabolomics further verified that 4,4-dimethylsterols influence unsaturated fatty acid biosynthesis and elevate oleoyl ethanolamine levels in the intestine. We propose a potential molecular mechanism in which 4,4-dimethylsterols engage in binding interactions with the catalytic pocket (Ser241) of FAAH-1 protein due to the shielded polarity, arising from the presence of 2 additional methyl groups (CH3). Consequently, 4,4-dimethylsterols represent an unexplored class of beneficial phytosterols that coordinate with FAAH-1 activity to reduce fat accumulation, which offers new insight into intervention strategies for treating diet-induced obesity.
Copyright © 2024 Tao Zhang et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Barreiro EJ, Kümmerle AE, Fraga CAM. The methylation effect in medicinal chemistry. Chem Rev. 2011;111(9):5215–5246. - PubMed
-
- Cheng X, Smith JC. Biological membrane organization and cellular signaling. Chem Rev. 2019;119(9):5849–5880. - PubMed
-
- He W-S, Li L-L, Huang Q-J, Yin J, Cao X-C. Highly efficient synthesis of phytosterol linolenate in the presence of Bronsted acidic ionic liquid. Food Chem. 2018;263:1–7. - PubMed
-
- Zhang T, Xie L, Liu R, Chang M, Zhang H, Jin Q, Wang X. Revisiting the 4,4-dimethylsterols profile from different kinds of vegetable oils by using GC-MS. LWT. 2020;124: Article 109163.
LinkOut - more resources
Full Text Sources
Research Materials

