Acinetobacter baumannii biofilm was inhibited by tryptanthrin through disrupting its different stages and genes expression
- PMID: 38812547
- PMCID: PMC11134903
- DOI: 10.1016/j.isci.2024.109942
Acinetobacter baumannii biofilm was inhibited by tryptanthrin through disrupting its different stages and genes expression
Abstract
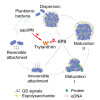
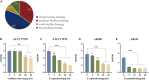
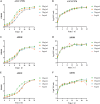
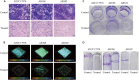
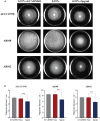
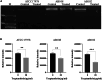
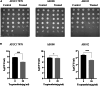
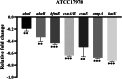
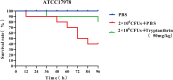
Biofilm formation plays a significant role in antibiotic resistance, necessitating the search for alternative therapies against biofilm-associated infections. This study demonstrates that 20 μg/mL tryptanthrin can hinder biofilm formation above 50% in various A. baumannii strains. Tryptanthrin impacts various stages of biofilm formation, including the inhibition of surface motility and eDNA release in A. baumannii, as well as an increase in its sensitivity to H202. RT-qPCR analysis reveals that tryptanthrin significantly decreases the expression of the following genes: abaI (19.07%), abaR (33.47%), bfmR (43.41%), csuA/B (64.16%), csuE (50.20%), ompA (67.93%), and katE (72.53%), which are related to biofilm formation and quorum sensing. Furthermore, tryptanthrin is relatively safe and can reduce the virulence of A. baumannii in a Galleria mellonella infection model. Overall, our study demonstrates the potential of tryptanthrin in controlling biofilm formation and virulence of A. baumannii by disrupting different stages of biofilm formation and intercellular signaling communication.
Keywords: Genetics; Microbiology; Molecular genetics.
© 2024 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Synergistic Inhibitory Effect of Polymyxin B in Combination with Ceftazidime against Robust Biofilm Formed by Acinetobacter baumannii with Genetic Deficiency in AbaI/AbaR Quorum Sensing.Microbiol Spectr. 2022 Feb 23;10(1):e0176821. doi: 10.1128/spectrum.01768-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196792 Free PMC article.
-
Contribution of the AbaI/AbaR Quorum Sensing System to Resistance and Virulence of Acinetobacter baumannii Clinical Strains.Infect Drug Resist. 2020 Nov 24;13:4273-4281. doi: 10.2147/IDR.S276970. eCollection 2020. Infect Drug Resist. 2020. PMID: 33262621 Free PMC article.
-
In vitro and in silico assessment of anti-biofilm and anti-quorum sensing properties of 2,4-Di-tert butylphenol against Acinetobacter baumannii.J Med Microbiol. 2024 Mar;73(3). doi: 10.1099/jmm.0.001813. J Med Microbiol. 2024. PMID: 38506718
-
Co-regulation of biofilm formation and antimicrobial resistance in Acinetobacter baumannii: from mechanisms to therapeutic strategies.Eur J Clin Microbiol Infect Dis. 2023 Dec;42(12):1405-1423. doi: 10.1007/s10096-023-04677-8. Epub 2023 Oct 28. Eur J Clin Microbiol Infect Dis. 2023. PMID: 37897520 Free PMC article. Review.
-
The role of quorum sensing, biofilm formation, and iron acquisition as key virulence mechanisms in Acinetobacter baumannii and the corresponding anti-virulence strategies.Microbiol Res. 2022 Jul;260:127032. doi: 10.1016/j.micres.2022.127032. Epub 2022 Apr 12. Microbiol Res. 2022. PMID: 35483311 Review.
Cited by
-
AbOmpA in Acinetobacter baumannii: exploring virulence mechanisms of outer membrane-integrated and outer membrane vesicle-associated AbOmpA and developing anti-infective agents targeting AbOmpA.J Biomed Sci. 2025 May 27;32(1):53. doi: 10.1186/s12929-025-01147-5. J Biomed Sci. 2025. PMID: 40426208 Free PMC article. Review.
-
A chionodracine-derived peptide, KHS-Cnd, as an anti-virulence agent against multidrug-resistant Acinetobacter baumannii clinical strains.Front Cell Infect Microbiol. 2025 Feb 14;15:1526246. doi: 10.3389/fcimb.2025.1526246. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40028178 Free PMC article.
-
Indole derivatives display antimicrobial and antibiofilm effects against extensively drug-resistant Acinetobacter baumannii.Microbiol Spectr. 2025 Apr 15;13(5):e0338824. doi: 10.1128/spectrum.03388-24. Online ahead of print. Microbiol Spectr. 2025. PMID: 40231681 Free PMC article.
-
Natural compounds for colistin-resistant Acinetobacter baumannii biofilm control: eugenol, cinnamaldehyde, and carvacrol.Mol Biol Rep. 2025 Jul 18;52(1):735. doi: 10.1007/s11033-025-10844-1. Mol Biol Rep. 2025. PMID: 40679553 No abstract available.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

