Nisin-based therapy: a realistic and eco-friendly biocontrol strategy to contrast Xylella fastidiosa subsp. pauca infections in planta
- PMID: 38812684
- PMCID: PMC11133578
- DOI: 10.3389/fmicb.2024.1406672
Nisin-based therapy: a realistic and eco-friendly biocontrol strategy to contrast Xylella fastidiosa subsp. pauca infections in planta
Abstract
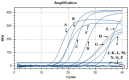
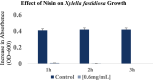
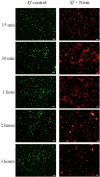
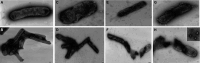
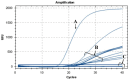
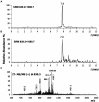
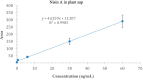
The lack of sustainable strategies for combating Xylella fastidiosa (Xf) highlights the pressing need for novel practical antibacterial tools. In this study, Lactococcus lactis subsp. lactis strain ATCC 11454 (L. lactis), known for its production of nisin A, was in vitro tested against Xf subsp. pauca. Preliminary investigations showed that nisin A was involved in a strong antagonistic activity exhibited by L. lactis against Xf. Thus, the efficacy of nisin A was comprehensively assessed through a combination of in vitro and in planta experiments. In vitro investigations employing viable-quantitative PCR, spot assay, turbidity reduction assay, fluorescence microscopy, and transmission electron microscopy demonstrated nisin's robust bactericidal effect on Xf at a minimal lethal concentration of 0.6 mg/mL. Moreover, results from fluorescence and transmission electron microscopies indicated that nisin directly and rapidly interacts with the membranes of Xf cells, leading to the destruction of bacterial cells in few minutes. In in planta tests, nisin also demonstrated the ability to tackle Xf infections within Nicotiana benthamiana plants that remained asymptomatic 74 days post inoculation. Furthermore, RPLC-ESI-MS/MS analyses showed that nisin translocated to all parts of the plants and remains intact for up to 9 days. For the first time, this study underscores the nisin-based strategy as a realistic and eco-friendly approach to be further investigated against Xf infections in the field.
Keywords: RPLC-ESI-MS/MS; antimicrobial peptide; bacteria; biocontrol; electron microscopy; v-qPCR.
Copyright © 2024 Sabri, El Handi, Valentini, De Stradis, Cara, Calvano, Bianco, Trani and Elbeaino.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Ali B. M., van der Werf W., Oude Lansink A. (2021). Assessment of the environmental impacts of Xylella fastidiosa subsp. pauca in Puglia. Crop Prot. 142:105519. doi: 10.1016/j.cropro.2020.105519 - DOI
-
- Ayivi R. D., Gyawali R., Krastanov A., Aljaloud S. O., Worku M., Tahergorabi R., et al. . (2020). Lactic acid bacteria: food safety and human health applications. Dairy 1, 202–232. doi: 10.3390/dairy1030015 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

