Multifaceted mitochondria in innate immunity
- PMID: 38812744
- PMCID: PMC11129950
- DOI: 10.1038/s44324-024-00008-3
Multifaceted mitochondria in innate immunity
Abstract
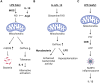
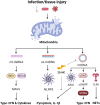
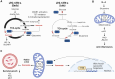
The ability of mitochondria to transform the energy we obtain from food into cell phosphorylation potential has long been appreciated. However, recent decades have seen an evolution in our understanding of mitochondria, highlighting their significance as key signal-transducing organelles with essential roles in immunity that extend beyond their bioenergetic function. Importantly, mitochondria retain bacterial motifs as a remnant of their endosymbiotic origin that are recognised by innate immune cells to trigger inflammation and participate in anti-microbial defence. This review aims to explore how mitochondrial physiology, spanning from oxidative phosphorylation (OxPhos) to signalling of mitochondrial nucleic acids, metabolites, and lipids, influences the effector functions of phagocytes. These myriad effector functions include macrophage polarisation, efferocytosis, anti-bactericidal activity, antigen presentation, immune signalling, and cytokine regulation. Strict regulation of these processes is critical for organismal homeostasis that when disrupted may cause injury or contribute to disease. Thus, the expanding body of literature, which continues to highlight the central role of mitochondria in the innate immune system, may provide insights for the development of the next generation of therapies for inflammatory diseases.
Keywords: Energy metabolism; Mitochondria.
© The Author(s) 2024.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
