mTOR pathway inhibition alters proliferation as well as differentiation of neural stem cells
- PMID: 38812794
- PMCID: PMC11133533
- DOI: 10.3389/fncel.2024.1298182
mTOR pathway inhibition alters proliferation as well as differentiation of neural stem cells
Abstract
Introduction: Neural stem cells (NSCs) are essential for both embryonic development and adult neurogenesis, and their dysregulation causes a number of neurodevelopmental disorders, such as epilepsy and autism spectrum disorders. NSC proliferation and differentiation in the developing brain is a complex process controlled by various intrinsic and extrinsic stimuli. The mammalian target of rapamycin (mTOR) regulates proliferation and differentiation, among other cellular functions, and disruption in the mTOR pathway can lead to severe nervous system development deficits. In this study, we investigated the effect of inhibition of the mTOR pathway by rapamycin (Rapa) on NSC proliferation and differentiation.
Methods: The NSC cultures were treated with Rapa for 1, 2, 6, 24, and 48 h. The effect on cellular functions was assessed by immunofluorescence staining, western blotting, and proliferation/metabolic assays.
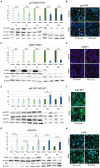
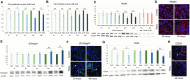
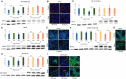
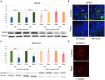
Results: mTOR inhibition suppressed NSC proliferation/metabolic activity as well as S-Phase entry by as early as 1 h of Rapa treatment and this effect persisted up to 48 h of Rapa treatment. In a separate experiment, NSCs were differentiated for 2 weeks after treatment with Rapa for 24 or 48 h. Regarding the effect on neuronal and glial differentiation (2 weeks post-treatment), this was suppressed in NSCs deficient in mTOR signaling, as evidenced by downregulated expression of NeuN, MAP2, and GFAP. We assume that the prolonged effect of mTOR inhibition is realized due to the effect on cytoskeletal proteins.
Discussion: Here, we demonstrate for the first time that the mTOR pathway not only regulates NSC proliferation but also plays an important role in NSC differentiation into both neuronal and glial lineages.
Keywords: cytoskeletal proteins; differentiation; mTOR; neural stem cells; proliferation.
Copyright © 2024 Romanyuk, Sintakova, Arzhanov, Horak, Gandhi, Jhanwar-Uniyal and Jendelova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
NOTCH Signaling Is Essential for Maturation, Self-Renewal, and Tri-Differentiation of In Vitro Derived Human Neural Stem Cells.Cell Reprogram. 2017 Dec;19(6):372-383. doi: 10.1089/cell.2017.0009. Epub 2017 Oct 16. Cell Reprogram. 2017. PMID: 29035086
-
Angiopoietin-2 induces the neuronal differentiation of mouse embryonic NSCs via phosphatidylinositol 3 kinase-Akt pathway-mediated phosphorylation of mTOR.Am J Transl Res. 2019 Mar 15;11(3):1895-1907. eCollection 2019. Am J Transl Res. 2019. PMID: 30972213 Free PMC article.
-
S6K Promotes Dopaminergic Neuronal Differentiation Through PI3K/Akt/mTOR-Dependent Signaling Pathways in Human Neural Stem Cells.Mol Neurobiol. 2016 Aug;53(6):3771-3782. doi: 10.1007/s12035-015-9325-9. Epub 2015 Jul 5. Mol Neurobiol. 2016. PMID: 26143260
-
Role of mTOR Complexes in Neurogenesis.Int J Mol Sci. 2018 May 22;19(5):1544. doi: 10.3390/ijms19051544. Int J Mol Sci. 2018. PMID: 29789464 Free PMC article. Review.
-
Total Ginsenoside Extract from Panax ginseng Enhances Neural Stem Cell Proliferation and Neuronal Differentiation by Inactivating GSK-3β.Chin J Integr Med. 2022 Mar;28(3):229-235. doi: 10.1007/s11655-021-3508-1. Epub 2022 Jan 27. Chin J Integr Med. 2022. PMID: 35084698
Cited by
-
An evolutionary medicine and life history perspective on aging and disease: Trade-offs, hyperfunction, and mismatch.Evol Med Public Health. 2025 May 15;13(1):111-124. doi: 10.1093/emph/eoaf010. eCollection 2025. Evol Med Public Health. 2025. PMID: 40574888 Free PMC article. Review.
-
Alcohol induces p53-mediated apoptosis in neural crest by stimulating an AMPK-mediated suppression of TORC1, S6K, and ribosomal biogenesis.Reprod Toxicol. 2024 Dec;130:108747. doi: 10.1016/j.reprotox.2024.108747. Epub 2024 Nov 7. Reprod Toxicol. 2024. PMID: 39521100
References
-
- Breuleux M., Klopfenstein M., Stephan C., Doughty C. A., Barys L., Maira S.-M., et al. . (2009). Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol. Cancer Ther. 8, 742–753. doi: 10.1158/1535-7163.MCT-08-0668, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

