Cold-evoked potentials in Fabry disease and polyneuropathy
- PMID: 38812855
- PMCID: PMC11133603
- DOI: 10.3389/fpain.2024.1352711
Cold-evoked potentials in Fabry disease and polyneuropathy
Abstract
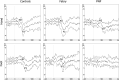
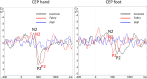
Background: Fabry disease (FD) causes cold-evoked pain and impaired cold perception through small fiber damage, which also occurs in polyneuropathies (PNP) of other origins. The integrity of thinly myelinated fibers and the spinothalamic tract is assessable by cold-evoked potentials (CEPs). In this study, we aimed to assess the clinical value of CEP by investigating its associations with pain, autonomic measures, sensory loss, and neuropathic signs.
Methods: CEPs were examined at the hand and foot dorsum of patients with FD (n = 16) and PNP (n = 21) and healthy controls (n = 23). Sensory phenotyping was performed using quantitative sensory testing (QST). The painDETECT questionnaire (PDQ), FabryScan, and measures for the autonomic nervous system were applied. Group comparisons and correlation analyses were performed.
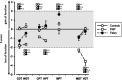
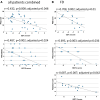
Results: CEPs of 87.5% of the FD and 85.7% of the PNP patients were eligible for statistical analysis. In all patients combined, CEP data correlated significantly with cold detection loss, PDQ items, pain, and autonomic measures. Abnormal CEP latency in FD patients was associated with an abnormal heart frequency variability item (r = -0.684; adjusted p = 0.04). In PNP patients, CEP latency correlated significantly with PDQ items, and CEP amplitude correlated with autonomic measures (r = 0.688, adjusted p = 0.008; r = 0.619, adjusted p = 0.024). Furthermore, mechanical pain thresholds differed significantly between FD (gain range) and PNP patients (loss range) (p = 0.01).
Conclusions: Abnormal CEPs were associated with current pain, neuropathic signs and symptoms, and an abnormal function of the autonomic nervous system. The latter has not been mirrored by QST parameters. Therefore, CEPs appear to deliver a wider spectrum of information on the sensory nervous system than QST alone.
Keywords: Fabry disease; cold-evoked potentials; diagnostic workup; neuropathic pain; neurophysiology; polyneuropathy.
© 2024 Kersebaum, Sendel, Lassen, Fabig, Forstenpointner, Reimer, Canaan-Kühl, Gaedeke, Rehm, Gierthmühlen, Baron and Hüllemann.
Conflict of interest statement
DK reports a grant, non-financial support, and a personal fee for a podcast episode from Grünenthal GmbH outside this study. MS is a consultant for Takeda Pharmaceutical and Merz Pharma; she reports personal fees from Alnylam Pharmaceuticals, Sanofi Genzyme, Grünenthal GmbH, Amicus Therapeutics, and Akcea, outside the submitted work. JL has received personal fees from Pfizer OFG Germany GmbH. S-CF reports grants from Grünenthal GmbH, during the conduct of this study as well as personal fees from Grünenthal GmbH outside the submitted work (speaker fees). Furthermore, she received financial support from Pfizer OFG Germany GmbH (personal fees). JF reports a grant (FO 1311/1-1) from the German Research Foundation (DFG); personal fees and non-financial support from Grünenthal GmbH and Sanofi Genzyme GmbH, personal fees from Bayer, non-financial support from Novartis, outside the submitted work. SC-K has received honoraria from Amicus Therapeutics, Chiesi Farmaceutici, Sanofi, and Takeda Pharmaceuticals. JGa reports grants from Amicus Therapeutics, Takeda Pharmaceuticals, and Sanofi Genzyme. JGi reports speaker fees from TAD Pharma, Insignia, Lilly GmbH, and Neurotech GmbH; consultant fees from Omega Pharma and Certkom; and travel support from Novartis, Lilly GmbH and Teva, outside the submitted work. RB reports grants/research support [EU Projects: “Europain“ (115007). DOLORisk (633491), IMI Paincare (777500), German Federal Ministry of Education and Research (BMBF): Verbundprojekt: Frühdetektion von Schmerzchronifizierung (NoChro) (13GW0338C), German Research Network on Neuropathic Pain (01EM0903), Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma Research GmbH und Co. KG., Novartis Pharma GmbH, Alnylam Pharmaceuticals Inc., Zambon GmbH, Sanofi-Aventis Deutschland GmbH]; speaker fees (Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma, Sanofi Pasteur, Medtronic Inc. Neuromodulation, Eisai Co. Ltd., Lilly GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Astellas Pharma GmbH, Desitin Arzneimittel GmbH, Teva GmbH, Bayer-Schering, MSD GmbH, Seqirus Australia Pty. Ltd., Novartis Pharma GmbH, TAD Pharma GmbH, Grünenthal SA Portugal, Sanofi-Aventis Deutschland GmbH, Agentur Brigitte Süss, Grünenthal Pharma AG Schweiz, Grünenthal B.V. Niederlande, Evapharma, Takeda Pharmaceuticals International AG Schweiz, Ology Medical Education Netherlands, Ever Pharma GmbH, Amicus Therapeutics GmbH); and consultant fees (Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma Research GmbH und Co. KG, Allergan, Sanofi Pasteur, Medtronic, Eisai, Lilly GmbH, Boehringer Ingelheim Pharma GmbH&Co. KG, Astellas Pharma GmbH, Novartis Pharma GmbH, Bristol Myers Squibb, Biogenidec, AstraZeneca GmbH, Merck, Abbvie, Daiichi Sankyo, Glenmark Pharmaceuticals S.A., Seqirus Australia Pty. Ltd., Teva Pharmaceuticals Europe Niederlande, Teva GmbH, Genentech, Mundipharma International Ltd. UK, Astellas Pharma Ltd. UK, Galapagos NV, Kyowa Kirin GmbH, Vertex Pharmaceuticals Inc., Biotest AG, Celgene GmbH, Desitin Arzneimittel GmbH, Regeneron Pharmaceuticals Inc. USA, Theranexus DSV CEA Frankreich, Abbott Products Operations AG Schweiz, Bayer AG, Grünenthal Pharma AG Schweiz, Mundipharma Research Ltd. UK, Akcea Therapeutics Germany GmbH, Asahi Kasei Pharma Corporation, AbbVie Deutschland GmbH & Co. KG, Air Liquide Sante International Frankreich, Alnylam Germany GmbH, Lateral Pharma Pty Ltd., Hexal AG, Angelini, Janssen, SIMR Biotech Pty Ltd. Australien, Confo Therapeutics N. V. Belgium, Merz Pharmaceuticals GmbH, Neumentum Inc., F. Hoffmann-La Roche Ltd. Switzerland, AlgoTherapeutix SAS France). PH reports grants from BMBF, Medoc, and Zambon outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. (2014) 155:2263–73. 10.1016/J.PAIN.2014.08.014 - DOI - PubMed
-
- Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1,236 patients with different neuropathic pain syndromes. Pain. (2010) 150:439–50. 10.1016/J.PAIN.2010.05.002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

