Emicizumab is well tolerated and effective in people with congenital hemophilia A regardless of age, severity of disease, or inhibitor status: a scoping review
- PMID: 38812987
- PMCID: PMC11135026
- DOI: 10.1016/j.rpth.2024.102415
Emicizumab is well tolerated and effective in people with congenital hemophilia A regardless of age, severity of disease, or inhibitor status: a scoping review
Abstract
Background: With the treatment landscape continually evolving, it is vital that the hemophilia community have an overview of all published data for approved therapies, such as emicizumab, to support shared decision making.
Objectives: To bring together the clinical and real-world data for emicizumab use in people with congenital hemophilia A, regardless of age, disease severity, or factor VIII inhibitor status. Key focus areas were safety, efficacy, and quality of life (QoL).
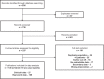
Methods: This scoping review used citation databases (PubMed, Embase, and the Cochrane Library) and manual searches of abstract books. Publications reporting original data for emicizumab in people with hemophilia A, published in English after December 2014, and reporting select endpoints were included. This narrative synthesis focused on zero bleeds, treated annualized bleeding rate (ABR), adverse events, and QoL measures.
Results: Overall, 97 publications were included (cut-off: August 9, 2022). Treated ABR remained low (calculated mean and median treated ABRs ranged between 0.7-1.3 and 0.0-1.4, respectively), and the median percentage of people with zero treated bleeds was 66.7%. The proportion of people experiencing treatment-related adverse events ranged from 0.0% to 60.0%; most were injection-site reactions. Across 37 publications reporting on safety and enrolling >2300 individuals, 11 thrombotic events and 4 thrombotic microangiopathies were reported. Data from well-established tools show QoL benefits with emicizumab.
Conclusion: This scoping review consolidates the global published experience for emicizumab in people with hemophilia A and supports the fact that emicizumab has an acceptable safety profile, is effective and efficacious in bleed prevention, and is associated with improvements in QoL.
Keywords: clinical trials; emicizumab; hemophilia A; quality of life; real-world experience; review; safety.
© 2024 The Authors.
Figures


References
-
- Mancuso M.E., Mahlangu J.N., Pipe S.W. The changing treatment landscape in haemophilia: from standard half-life clotting factor concentrates to gene editing. Lancet. 2021;397:630–640. - PubMed
-
- von Drygalski A., Chowdary P., Kulkarni R., Susen S., Konkle B.A., Oldenburg J., et al. Efanesoctocog alfa prophylaxis for patients with severe hemophilia A. N Engl J Med. 2023;388:310–318. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

