A critical mini-review on doping and heterojunction formation in ZnO-based catalysts
- PMID: 38813127
- PMCID: PMC11134265
- DOI: 10.1039/d4ra02568g
A critical mini-review on doping and heterojunction formation in ZnO-based catalysts
Abstract
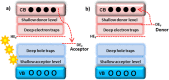
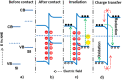
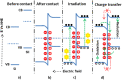
This mini-review on doping and heterojunctions for catalysis applications provides a comprehensive overview of key aspects. Doping, when carried out adequately with a uniform distribution, creates a new energy level that significantly enhances charge transfer and light absorption. This new level alters the material's morphology and enhances intrinsic defects. For instance, ZnO, despite its exceptional band edge concerning oxygen reduction and water oxidation redox potentials, faces the issue of electron-hole recombination. However, forming a heterojunction can effectively aid charge transfer and prolong electron-hole relaxation without recombination. This is where the role of doping and heterojunctions becomes crucial. Additionally, incorporating noble metals with S- and Z-scheme heterojunctions offers a promising mechanism for charge transfer and visible light harvesting, further amplifying the catalytic properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Ali S. Ismail P. M. Khan M. Dang A. Ali S. Zada A. Raziq F. Khan I. Khan M. S. Ateeq M. Khan W. Bakhtiar S. H. Ali H. Wu X. Shah M. I. A. Vinu A. Yi J. Xia P. Qiao L. Nanoscale. 2024;16:4352–4377. - PubMed
-
- Song Y. Mendes P. C. D. Kozlov S. M. J. Mater. Chem. A. 2023;11:13665–13676.
-
- Debnath D. Sen D. Neog T. T. Saha B. Ghosh S. K. Cryst. Growth Des. 2024;24:871–885.
-
- Thennarasu G. Sivasamy A. Powder Technol. 2013;250:1–12.
-
- Peng Y. Qin S. Wang W.-S. Xu A.-W. CrystEngComm. 2013;15:6518.
Publication types
LinkOut - more resources
Full Text Sources

