Multiple dyes applications for fluorescent convertible polymer capsules as macrophages tracking labels
- PMID: 38813172
- PMCID: PMC11133507
- DOI: 10.1016/j.heliyon.2024.e30680
Multiple dyes applications for fluorescent convertible polymer capsules as macrophages tracking labels
Abstract
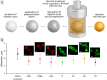
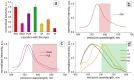
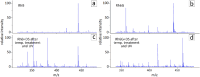
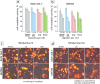
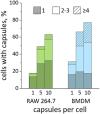
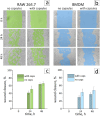
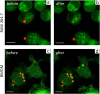
Tracing individual cell pathways among the whole population is crucial for understanding their behavior, cell communication, migration dynamics, and fate. Optical labeling is one approach for tracing individual cells, but it typically requires genetic modification to induce the generation of photoconvertible proteins. Nevertheless, this approach has limitations and is not applicable to certain cell types. For instance, genetic modification often leads to the death of macrophages. This study aims to develop an alternative method for labeling macrophages by utilizing photoconvertible micron-sized capsules capable of easy internalization and prolonged retention within cells. Thermal treatment in a polyvinyl alcohol gel medium is employed for the scalable synthesis of capsules with a wide range of fluorescent dyes, including rhodamine 6G, pyronin B, fluorescein, acridine yellow, acridine orange, thiazine red, and previously reported rhodamine B. The fluorescence brightness, photostability, and photoconversion ability of the capsules are evaluated using confocal laser scanning microscopy. Viability, uptake, mobility, and photoconversion studies are conducted on RAW 264.7 and bone marrow-derived macrophages, serving as model cell lines. The production yield of the capsules is increased due to the use of polyvinyl alcohol gel, eliminating the need for conventional filtration steps. Capsules entrapping rhodamine B and rhodamine 6G meet all requirements for intracellular use in individual cell tracking. Mass spectrometry analysis reveals a sequence of deethylation steps that result in blue shifts in the dye spectra upon irradiation. Cellular studies on macrophages demonstrate robust uptake of the capsules. The capsules exhibit minimal cytotoxicity and have a negligible impact on cell motility. The successful photoconversion of RhB-containing capsules within cells highlights their potential as alternatives to photoconvertible proteins for individual cell labeling, with promising applications in personalized medicine.
Keywords: Carbon nanoparticle; Cell imaging; Cell labeling; Deethylation; Encapsulation; Fluorescent label; Macrophage; Microcapsule; Photoconversion; Photoconvertible label.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Single Mesenchymal Stromal Cell Migration Tracking into Glioblastoma Using Photoconvertible Vesicles.Nanomaterials (Basel). 2024 Jul 17;14(14):1215. doi: 10.3390/nano14141215. Nanomaterials (Basel). 2024. PMID: 39057891 Free PMC article.
-
Labeling and Tracking of Individual Human Mesenchymal Stromal Cells Using Photoconvertible Fluorescent Microcapsules.Int J Mol Sci. 2023 Sep 4;24(17):13665. doi: 10.3390/ijms241713665. Int J Mol Sci. 2023. PMID: 37686471 Free PMC article.
-
Effect of photoconversion conditions on the spectral and cytotoxic properties of photoconvertible fluorescent polymer markers.Phys Chem Chem Phys. 2024 May 1;26(17):13078-13086. doi: 10.1039/d3cp04606k. Phys Chem Chem Phys. 2024. PMID: 38628110
-
Fluorescent Convertible Capsule Coding Systems for Individual Cell Labeling and Tracking.ACS Appl Mater Interfaces. 2021 May 5;13(17):19701-19709. doi: 10.1021/acsami.1c02767. Epub 2021 Apr 26. ACS Appl Mater Interfaces. 2021. PMID: 33900738
-
Functionalized silica nanoparticles: a platform for fluorescence imaging at the cell and small animal levels.Acc Chem Res. 2013 Jul 16;46(7):1367-76. doi: 10.1021/ar3001525. Epub 2013 Mar 14. Acc Chem Res. 2013. PMID: 23489227 Review.
Cited by
-
Long-term tracing of individual human neural cells using multiphoton microscopy and photoconvertible polymer capsules.J R Soc Interface. 2024 Oct;21(219):20240497. doi: 10.1098/rsif.2024.0497. Epub 2024 Oct 30. J R Soc Interface. 2024. PMID: 39471872
-
Single Mesenchymal Stromal Cell Migration Tracking into Glioblastoma Using Photoconvertible Vesicles.Nanomaterials (Basel). 2024 Jul 17;14(14):1215. doi: 10.3390/nano14141215. Nanomaterials (Basel). 2024. PMID: 39057891 Free PMC article.
References
LinkOut - more resources
Full Text Sources

