Evaluation of two alternative non-alcohol-based media for the suspension of self-collected vaginal swabs for HPV testing in cervical cancer screening
- PMID: 38813186
- PMCID: PMC11133750
- DOI: 10.1016/j.heliyon.2024.e31032
Evaluation of two alternative non-alcohol-based media for the suspension of self-collected vaginal swabs for HPV testing in cervical cancer screening
Abstract
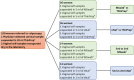
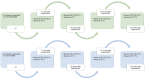
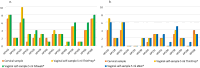
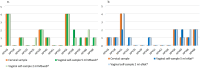
The introduction of Human Papillomavirus (HPV) testing in cervical cancer screening enhanced the opportunity to introduce self-collection as an innovative approach to improve coverage rates. Validation and standardization of the pre-analytical and analytical procedures are crucial for the quality assurance of HPV tests on self-collected samples. This study evaluated the analytical performance and the stability of self-collected vaginal samples resuspended in 5 mL of two non-alcohol-based media, eNat® and MSwab® compared to a professionally collected cervical sample, resuspended in 20 mL ThinPrep®, for the detection of high-risk HPV (hrHPV). The impact of the suspension volumes on analytical performance was also evaluated (2 and 5 ml). A good analytical concordance in hrHPV detection in cervical and vaginal self-collected swabs suspended in 5 ml of both non-alcohol-based media was demonstrated (eNat®: 91.2 %, k = 0.821; MSwab®: 91.4 %; k = 0.798). A similar analytical performance was found for samples resuspended in 2 mL (eNat®: 92.9 %, k = 0.811; MSwab®: 92.9 %, k = 0.811) compared to cervical samples. Good nucleic acid stability was demonstrated for vaginal samples stored at 20-25 °C and 37 °C for up to 4 weeks. Results of this preliminary study support the introduction of these media for vaginal self-sampling-based prevention programs. Nevertheless, further research is necessary to evaluate clinical accuracy in larger settings.
Keywords: Cervical cancer; HPV; HPV testing; Human papillomavirus; Self-sampling; Suspension medium.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Clementina Elvezia Cocuzza received research funding or gratis consumables to support research from the following commercial entities in the last 3 years: BD, Seegene, Copan, Novosanis and Hiantis. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Bouvard V., Wentzensen N., Mackie A., Berkhof J., Brotherton J., Giorgi-Rossi P., Kupets R., Smith R., Arrossi S., Bendahhou K., Canfell K., Chirenje Z.M., Chung M.H., Del Pino M., de Sanjosé S., Elfström M., Franco E.L., Hamashima C., Hamers F.F., Herrington C.S., Murillo R., Sangrajrang S., Sankaranarayanan R., Saraiya M., Schiffman M., Zhao F., Arbyn M., Prendiville W., Indave Ruiz B.I., Mosquera-Metcalfe I., Lauby-Secretan B. The IARC perspective on cervical cancer screening. N. Engl. J. Med. 2021 Nov 11;385(20):1908–1918. doi: 10.1056/NEJMsr2030640. PMID: 34758259. - DOI - PubMed
-
- Kyrgiou M., Arbyn M., Bergeron C., Bosch F.X., Dillner J., Jit M., Kim J., Poljak M., Nieminen P., Sasieni P., Kesic V., Cuzick J., Gultekin M. Cervical screening: ESGO-EFC position paper of the European society of gynaecologic oncology (ESGO) and the European federation of colposcopy (EFC) Br. J. Cancer. 2020 Aug;123(4):510–517. doi: 10.1038/s41416-020-0920-9. Epub 2020 Jun 8. PMID: 32507855; PMCID: PMC7434873. - DOI - PMC - PubMed
-
- Arbyn M., Castle P.E., Schiffman M., Wentzensen N., Heckman-Stoddard B., Sahasrabuddhe V.V. Meta-analysis of agreement/concordance statistics in studies comparing self- versus clinician-collected samples for HPV testing in cervical cancer screening. Int. J. Cancer. 2022 Feb;18 doi: 10.1002/ijc.33967. Epub ahead of print. PMID: 35179777. - DOI - PubMed
-
- Arbyn M., Smith S.B., Temin S., Sultana F., Castle P. Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018 Dec 5;363:k4823. doi: 10.1136/bmj.k4823. PMID: 30518635; PMCID: PMC6278587. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

