Deciphering the Efficacy of β-Lactams in the Face of Metallo-β-Lactamase-Derived Resistance in Enterobacterales: Supraphysiologic Zinc in the Broth Is the Culprit
- PMID: 38813259
- PMCID: PMC11134298
- DOI: 10.1093/ofid/ofae228
Deciphering the Efficacy of β-Lactams in the Face of Metallo-β-Lactamase-Derived Resistance in Enterobacterales: Supraphysiologic Zinc in the Broth Is the Culprit
Abstract
Background: In vitro-in vivo discordance in β-lactams' activities against metallo-ß-lactamase (MBL)-producing Enterobacterales has been described. We aimed to assess whether this discordance is attributed to the supra-physiologic zinc concentration in in vitro testing media.
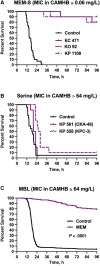
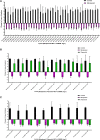
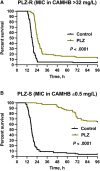
Methods: A clinical and microbiological observational study of patients with bloodstream infections due to New Delhi metallo-ß-lactamase-producing Klebsiella pneumoniae was performed. Outcomes of patients treated empirically with non-MBL-active β-lactam therapy (carbapenems and ceftazidime/avibactam) and MBL-active β-lactam therapy (ceftazidime/avibactam + aztreonam) were documented. The patients' isolates were used to induce septicemia in mice, and survival upon meropenem treatment was recorded. Meropenem minimum inhibitory concentrations (MICs) were determined in standard media and in the presence of physiological zinc concentrations.
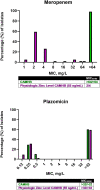
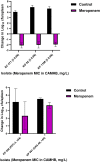
Results: Twenty-nine patients receiving empiric non-MBL-active β-lactams (median duration, 4 days) were compared with 29 receiving MBL-active β-lactams. The 14-day mortality rates were 21% and 14%, respectively. In the murine septicemia model, meropenem treatment resulted in protection from mortality (P < .0001). Meropenem MICs in the physiologic zinc concentration broth were 1- to >16-fold lower vs MICs in zinc-unadjusted broth (≥64 mg/L).
Conclusions: Our data provide foundational support to establish pharmacokinetic/pharmacodynamic relationships using MICs derived in physiologic zinc concentration, which may better predict β-lactam therapy outcome.
Keywords: Klebsiella pneumoniae; NDM; bloodstream infections; carbapenem; zinc.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflict of interest. K.A. has received research grants from the FDA, Evopoint Biosciences, Venatorx Pharmaceuticals, and Toscana Life Sciences Foundation. C.M.G. received research funding from Cepheid, Everest Medicines, Shionogi, and Entasis. M.F. received unconditional grants from MSD and grants or speaker honoraria from Angelini, Shionogi, Pfizer, Menarini, Gilead, and Nordic Pharma. G.T. received honoraria for educational meetings for Shionogi. D.P.N. is a consultant, speaker bureau member, and has received other research grants from AbbVie, Cepheid, Merck, Paratek, Pfizer, Wockhardt, and Shionogi. All other authors report no potential conflicts. Declared conflicts of interest are outside the submitted work and did not affect the scientific objectivity of this study.
Figures
Similar articles
-
Activity of cefiderocol and synergy of novel β-lactam-β-lactamase inhibitor-based combinations against metallo-β-lactamase-producing gram-negative bacilli: insights from a two-year study (2019-2020).J Chemother. 2023 May;35(3):198-204. doi: 10.1080/1120009X.2022.2090615. Epub 2022 Jun 22. J Chemother. 2023. PMID: 35731718
-
In vitro activity of aztreonam-avibactam against Enterobacterales isolates collected in Latin America, Africa/Middle East, Asia, and Eurasia for the ATLAS Global Surveillance Program in 2019-2021.Eur J Clin Microbiol Infect Dis. 2023 Sep;42(9):1135-1143. doi: 10.1007/s10096-023-04645-2. Epub 2023 Aug 1. Eur J Clin Microbiol Infect Dis. 2023. PMID: 37526796 Free PMC article.
-
The paradoxical in vivo activity of β-lactams against metallo-β-lactamase-producing Enterobacterales is not restricted to carbapenems.J Antimicrob Chemother. 2021 Feb 11;76(3):684-691. doi: 10.1093/jac/dkaa467. J Antimicrob Chemother. 2021. PMID: 33179050
-
New β-Lactam-β-Lactamase Inhibitor Combinations.Clin Microbiol Rev. 2020 Nov 11;34(1):e00115-20. doi: 10.1128/CMR.00115-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33177185 Free PMC article. Review.
-
The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases.Antibiotics (Basel). 2021 Aug 20;10(8):1012. doi: 10.3390/antibiotics10081012. Antibiotics (Basel). 2021. PMID: 34439062 Free PMC article. Review.
Cited by
-
Efficacy of imipenem combined with dimercaptosuccinic acid in a murine sepsis model using Pseudomonas aeruginosa.Sci Rep. 2025 Aug 1;15(1):28047. doi: 10.1038/s41598-025-13554-7. Sci Rep. 2025. PMID: 40745035 Free PMC article.
References
-
- Chibabhai V, Nana T, Bosman N, Thomas T, Lowman W. Were all carbapenemases created equal? Treatment of NDM-producing extensively drug-resistant Enterobacteriaceae: a case report and literature review. Infection 2018; 46:1–13. - PubMed
-
- Kohler PP, Volling C, Green K, Uleryk EM, Shah PS, McGeer A. Carbapenem resistance, initial antibiotic therapy, and mortality in Klebsiella pneumoniae bacteremia: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2017; 38:1319–28. - PubMed
-
- Mura M, Longo B, Andreini R, et al. Clinical outcomes in elderly patients with infections caused by NDM-producing Klebsiella pneumoniae: results from a real-life retrospective single center study in an endemic area. Intern Emerg Med 2023; 18:2261–9. - PubMed
-
- Asempa TE, Abdelraouf K, Nicolau DP. Metallo-β-lactamase resistance in Enterobacteriaceae is an artefact of currently utilized antimicrobial susceptibility testing methods. J Antimicrob Chemother 2020; 75:997–1005. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

