Dietary supplement for mood symptoms in early postpartum: a double-blind randomized placebo controlled trial
- PMID: 38813444
- PMCID: PMC11133796
- DOI: 10.1016/j.eclinm.2024.102593
Dietary supplement for mood symptoms in early postpartum: a double-blind randomized placebo controlled trial
Abstract
Background: Postpartum blues (PPB) is a frequent syndrome of sad mood, crying spells, anxiety, restlessness, reduced appetite, and irritability, typically peaking day 5 postpartum. When severe, it greatly increases risk for later postpartum depression. This trial compared a dietary supplement to placebo on PPB severity. The supplement was designed to counter downstream effects of elevated monoamine oxidase A level, implicated in causing PPB.
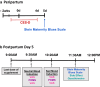
Methods: Participants recruited by advertisement from the Toronto region completed procedures at CAMH, Canada and/or participants' homes. Oral supplement or identical appearing relatively inert placebo were administered in randomised, double-blind fashion. Supplement was blueberry juice and extract given four times between nighttime day 3 and morning day 5 postpartum; tryptophan 2 g nighttime day 4 postpartum, and tyrosine 10 g morning day 5 postpartum. On day 5, depressed mood induction procedure (MIP) and postpartum blues were assessed. All data is presented (NCT03296956 closed, clinicaltrials.gov).
Findings: Between January 2019 and December 2022, participants took supplement (n = 51) or placebo (n = 52). There was no significant effect on primary outcome MIP on visual analogue scale for depressed mood (mean difference = -0.39 mm, 95% CI: -6.42 to 5.65 mm). Stein Maternity Blues scores, exploratory PPB measure, was lower in the active group (effect size 0.62; median, interquartile range (IQR): active 2.00 (IQR 1, 4); placebo 4.00 (IQR 1.5, 6); regression with general linear model, supplement effect, β coefficient = -1.50 (95%: CI -2.60, -0.40), p = 0.008; effect of CES-D crying category before supplement, p = 0.03-0.00000023). Twenty-six and 40 different adverse events occurred within 25% and 42% of supplement and placebo cases respectively (Chi-Square, p = 0.06).
Interpretation: The primary outcome was negative for effect on depressed mood induction, however the supplement moderately reduced PPB.
Funding: CAMH/Exeltis.
Keywords: Antioxidants; Postpartum blues; Postpartum depression; Tryptophan; Tyrosine.
© 2024 The Authors.
Conflict of interest statement
JHM is the inventor on patents for the dietary supplement and there is an agreement between CAMH and Exeltis for the latter to manufacture, and distribute the dietary supplement. JHM also has patents for blood biomarkers in mood disorders to predict neuroinflammation, elevated MAO-B level and elevated MAO-A level in the brain. JHM has received operating grant funding from Exeltis and Sanofi in the past 2 years. MIH receives research grants from the Canadian Institutes of Health Research, CAMH Foundation, University of Toronto, COMPASS Pathfinder; stipend from Society of Biological Psychiatry; payment or honoraria from the American Society of Clinical Psychopharmacology and American College Health Association; consulting fees from Wake Network Inc. and stock options in Mindset Pharma Inc.
Figures



Similar articles
-
Selective dietary supplementation in early postpartum is associated with high resilience against depressed mood.Proc Natl Acad Sci U S A. 2017 Mar 28;114(13):3509-3514. doi: 10.1073/pnas.1611965114. Epub 2017 Mar 13. Proc Natl Acad Sci U S A. 2017. PMID: 28289215 Free PMC article. Clinical Trial.
-
Depressed mood induction in early cigarette withdrawal is unaffected by acute monoamine precursor supplementation.Neuropsychiatr Dis Treat. 2019 Jan 23;15:311-321. doi: 10.2147/NDT.S172334. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 30774343 Free PMC article.
-
Effect of dysfunctional attitudes and postpartum state on vulnerability to depressed mood.J Affect Disord. 2014 Jun;161:16-20. doi: 10.1016/j.jad.2014.02.047. Epub 2014 Mar 12. J Affect Disord. 2014. PMID: 24751302
-
Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults.Cochrane Database Syst Rev. 2021 Jan 18;1(1):CD013011. doi: 10.1002/14651858.CD013011.pub2. Cochrane Database Syst Rev. 2021. PMID: 33460048 Free PMC article.
-
Nutritional interventions for treating foot ulcers in people with diabetes.Cochrane Database Syst Rev. 2020 Jul 17;7(7):CD011378. doi: 10.1002/14651858.CD011378.pub2. Cochrane Database Syst Rev. 2020. PMID: 32677037 Free PMC article.
References
-
- O'Hara M.W., Schlechte J.A., Lewis D.A., Wright E.J. Prospective study of postpartum blues. Biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801–806. - PubMed
-
- Adewuya A.O. Early postpartum mood as a risk factor for postnatal depression in Nigerian women. Am J Psychiatry. 2006;163(8):1435–1437. - PubMed
-
- Hannah P., Adams D., Lee A., Glover V., Sandler M. Links between early post-partum mood and post-natal depression. Br J Psychiatry. 1992;160:777–780. - PubMed
-
- American_Psychiatric_Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. American psychiatric association: diagnostic and statistical manual of mental disorders.
-
- Sacher J., Chechko N., Dannlowski U., Walter M., Derntl B. The peripartum human brain: current understanding and future perspectives. Front Neuroendocrinol. 2020;59 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

