Vosoritide treatment for children with hypochondroplasia: a phase 2 trial
- PMID: 38813446
- PMCID: PMC11133798
- DOI: 10.1016/j.eclinm.2024.102591
Vosoritide treatment for children with hypochondroplasia: a phase 2 trial
Abstract
Background: Hypochondroplasia is a rare autosomal dominant skeletal dysplasia due to activating variants in FGFR3. It presents with disproportionate short stature with a wide range of clinical severity. There are currently no approved medications to treat short stature in children with hypochondroplasia. Vosoritide is a C-type natriuretic peptide analog that was recently approved for improving growth in children with achondroplasia. We aimed to evaluate the safety and efficacy of vosoritide in children with hypochondroplasia.
Methods: We conducted a single-arm, phase 2, open-label trial at a single centre in the USA and enrolled 26 children with hypochondroplasia. The trial consists of a 6-month observation period to establish a baseline annualized growth velocity followed by a 12-month intervention period during which vosoritide is administered daily via subcutaneous injection at a dose of 15 μg/kg/day. The trial's co-primary endpoints included the incidence of adverse events and the change from baseline in age-sex standardized annualized growth velocity and height standardized deviation score (SDS) after 12 months of treatment. This trial is registered with ClinicalTrials.gov (NCT04219007).
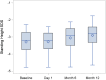
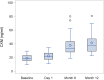
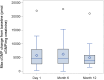
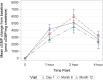
Findings: Twenty-four participants with a mean age of 5.86 years received vosoritide therapy. The first participant was enrolled on August 4, 2020, and the final participant completed the 18-month trial on September 8, 2023. Vosoritide was well tolerated with no treatment-related serious adverse events. Injection site reactions occurred in 83.3% of participants. No participants discontinued therapy due to an adverse event. Annualized growth velocity increased by 2.26 standard deviations (SD) and height SDS increased by 0.36 SD during the treatment period versus the observation period. Hypochondroplasia specific height SDS increased by 0.38 SD. There was a 1.81 cm/year increase in absolute annualized growth velocity.
Interpretation: Vosoritide was safe and effective in increasing growth velocity in children with hypochondroplasia. Efficacy was similar to what has been reported in children with achondroplasia.
Funding: This study was supported by an investigator-initiated grant from BioMarin Pharmaceutical.
Keywords: C-type natriuretic peptide; CNP; FGFR3; Hypochondroplasia; Vosoritide.
© 2024 The Author(s).
Conflict of interest statement
AD and NM have served as consultants for BioMarin, but all compensation has been paid to Children's National Hospital and neither author has received any personal compensation from BioMarin. AD received an investigator-initiated grant from BioMarin to fund the current study. RKS has received an investigator-initiated grant from BioMarin to fund a study of vosoritide in girls with Turner syndrome. The remaining authors have nothing to disclose.
Figures
References
-
- Bober M.B., Bellus G.A., Nikkel S.M., Tiller G.E. Hypochondroplasia. GeneReviews®. 1993. http://www.ncbi.nlm.nih.gov/pubmed/19622626
Associated data
LinkOut - more resources
Full Text Sources
Medical