Decoding trends in mRNA vaccine research: A comprehensive bibliometric study
- PMID: 38813652
- PMCID: PMC11141478
- DOI: 10.1080/21645515.2024.2355037
Decoding trends in mRNA vaccine research: A comprehensive bibliometric study
Abstract
Background: In recent years, infectious diseases like COVID-19 have had profound global socio-economic impacts. mRNA vaccines have gained prominence due to their rapid development, industrial adaptability, simplicity, and responsiveness to new variants. Notably, the 2023 Nobel Prize in Physiology or Medicine recognized significant contributions to mRNA vaccine research.
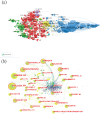
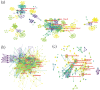
Methods: Our study employed a comprehensive bibliometric analysis using the Web of Science Core Collection (WoSCC) database, encompassing 5,512 papers on mRNA vaccines from 2003 to 2023. We generated cooperation maps, co-citation analyses, and keyword clustering to evaluate the field's developmental history and achievements.
Results: The analysis yielded knowledge maps highlighting countries/institutions, influential authors, frequently published and highly cited journals, and seminal references. Ongoing research hotspots encompass immune responses, stability enhancement, applications in cancer prevention and treatment, and combating infectious diseases using mRNA technology.
Conclusions: mRNA vaccines represent a transformative development in infectious disease prevention. This study provides insights into the field's growth and identifies key research priorities, facilitating advancements in vaccine technology and addressing future challenges.
Keywords: 2023 Nobel prize; bibliometric analysis; hotspot; mRNA vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
-
- Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo R, Bica MA, Garofano A, Koch SD. et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390(10101):1511–20. doi: 10.1016/S0140-6736(17)31665-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
