The glutamate/aspartate transporter EAAT1 is crucial for T-cell acute lymphoblastic leukemia proliferation and survival
- PMID: 38813748
- PMCID: PMC11532688
- DOI: 10.3324/haematol.2023.283471
The glutamate/aspartate transporter EAAT1 is crucial for T-cell acute lymphoblastic leukemia proliferation and survival
Abstract
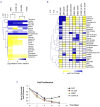
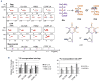
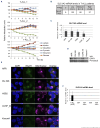
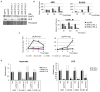
T-cell acute lymphoblastic leukemia (T-ALL) is a cancer of the immune system. Approximately 20% of pediatric and 50% of adult T-ALL patients have refractory disease or relapse and die from the disease. To improve patient outcome new therapeutics are needed. With the aim to identify new therapeutic targets, we combined the analysis of T-ALL gene expression and metabolism to identify the metabolic adaptations that T-ALL cells exhibit. We found that glutamine uptake is essential for T-ALL proliferation. Isotope tracing experiments showed that glutamine fuels aspartate synthesis through the tricarboxylic acid cycle and that glutamine and glutamine-derived aspartate together supply three nitrogen atoms in purines and all but one atom in pyrimidine rings. We show that the glutamate-aspartate transporter EAAT1 (SLC1A3), which is normally expressed in the central nervous system, is crucial for glutamine conversion to aspartate and nucleotides and that T-ALL cell proliferation depends on EAAT1 function. Through this work, we identify EAAT1 as a novel therapeutic target for T-ALL treatment.
Figures



References
-
- Homminga I, Pieters R, Langerak AW, et al. . Integrated transcript and genome analyses reveal NKX2-1 and MEF2C as potential oncogenes in T cell acute lymphoblastic leukemia. Cancer Cell. 2011;19(4):484-497. - PubMed
-
- Chessells JM, Veys P, Kempski H, et al. . Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123(3):396-405. - PubMed
-
- Vora A, Wade R, Mitchell CD, Goulden N, Richards S. Improved outcome for children and young adults with T-cell acute lymphoblastic leukaemia (ALL): results of the United Kingdom Medical Research Council (MRC) Trial UKALL 2003. Blood. 2008; 112(11):908.
-
- Hastings C, Gaynon PS, Nachman JB, et al. . Increased post-induction intensification improves outcome in children and adolescents with a markedly elevated white blood cell count (>/=200 x 10(9) /l) with T cell acute lymphoblastic leukaemia but not B cell disease: a report from the Children’s Oncology Group. Br J Haematol. 2015;168(4):533-546. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

