Surface Acoustic Wave-Driven Enhancement of Enzyme-Linked Immunosorbent Assays: ELISAW
- PMID: 38813952
- PMCID: PMC11170557
- DOI: 10.1021/acs.analchem.4c01615
Surface Acoustic Wave-Driven Enhancement of Enzyme-Linked Immunosorbent Assays: ELISAW
Abstract
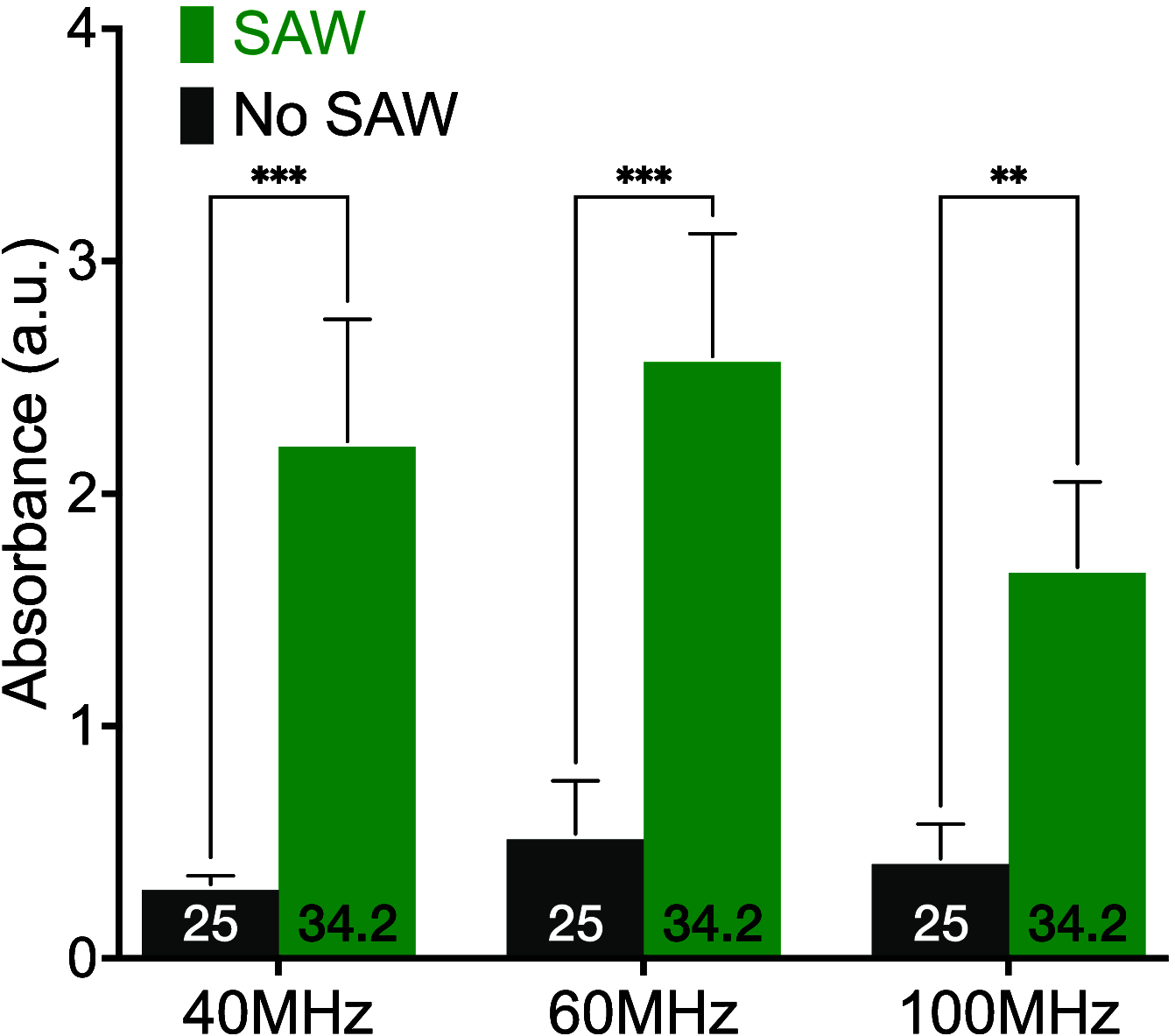
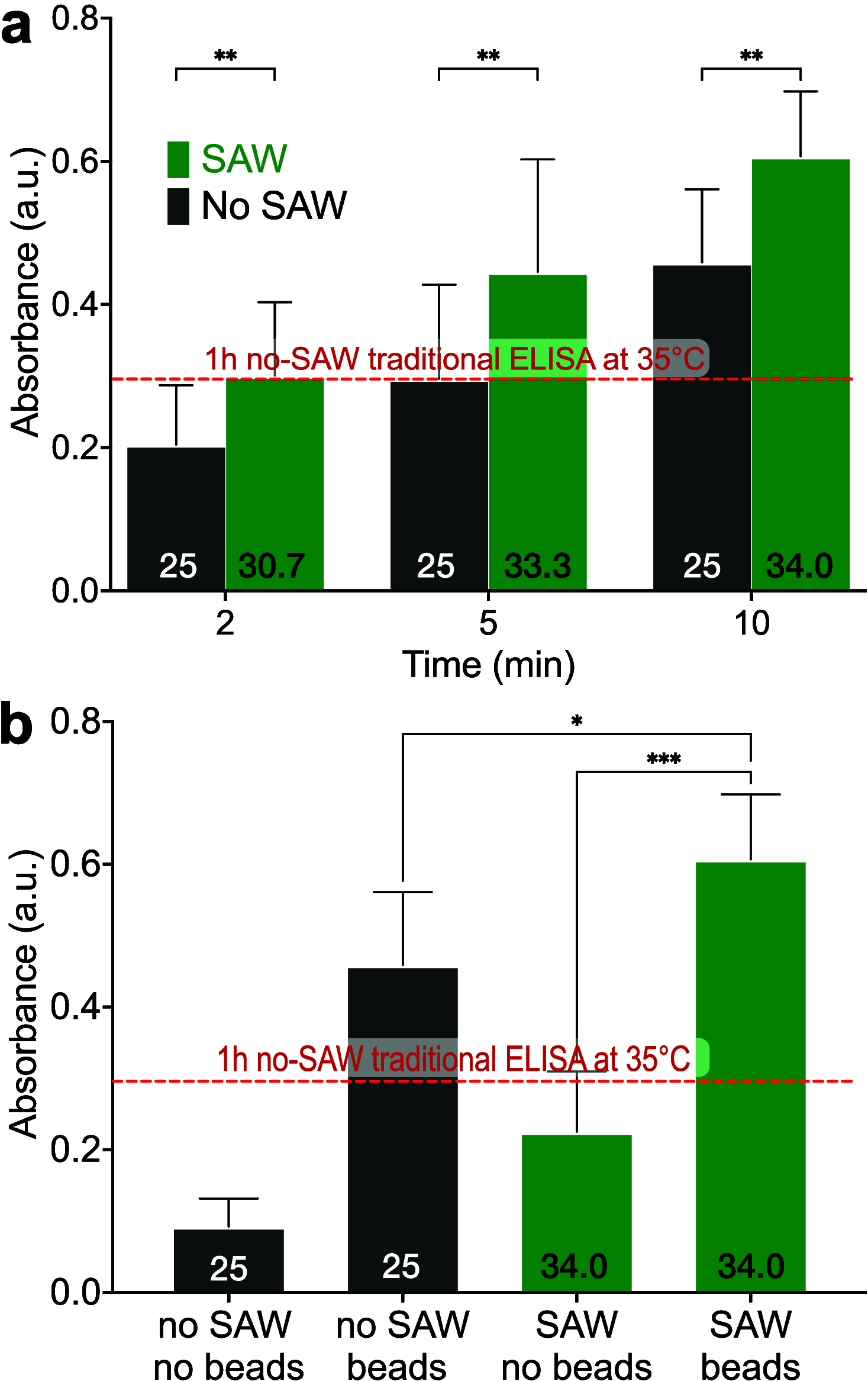
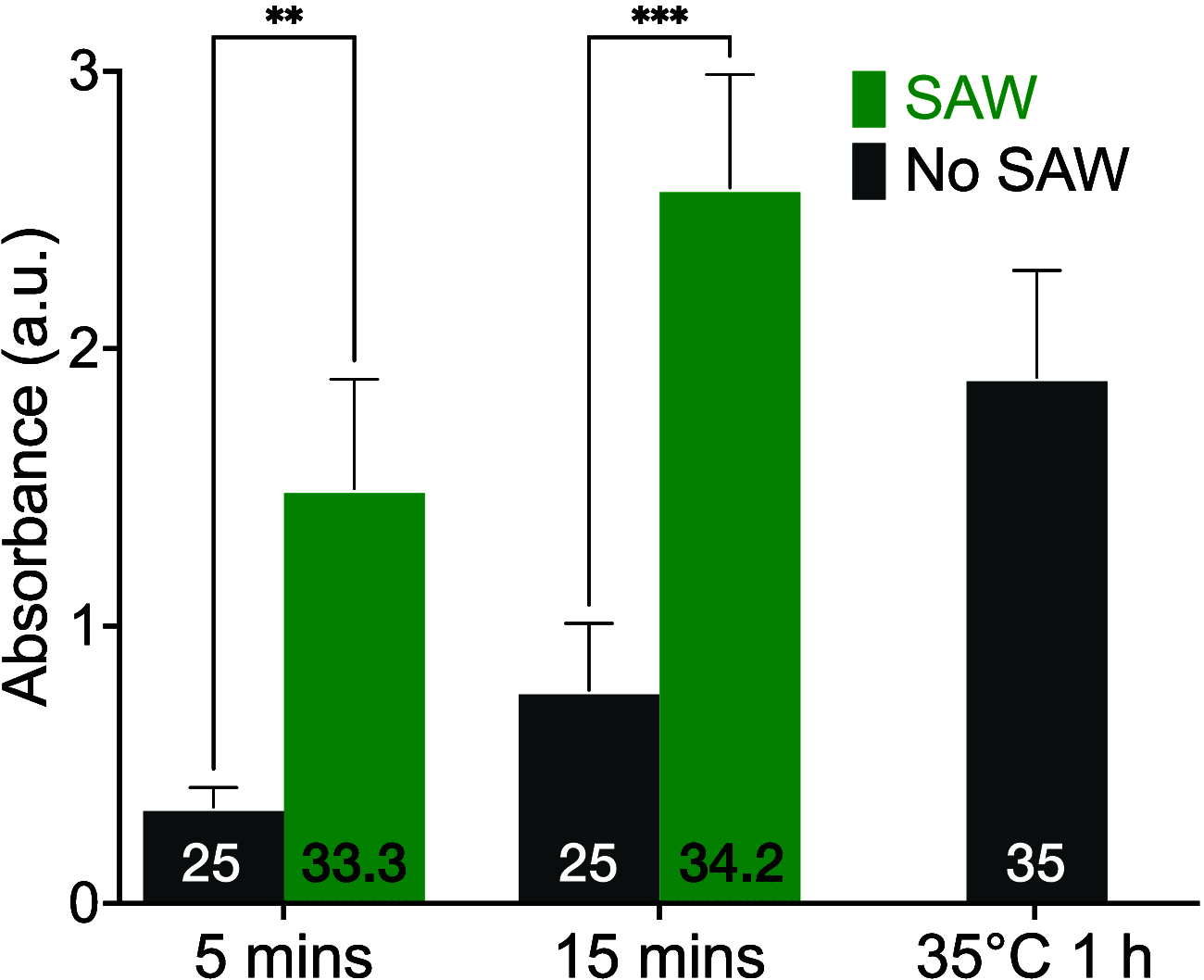
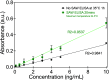
Enzyme-linked immunosorbent assays (ELISAs) are widely used in biology and clinical diagnosis. Relying on antigen-antibody interaction through diffusion, the standard ELISA protocol can be time-consuming, preventing its use in rapid diagnostics. We present a time-saving and more sensitive ELISA without changing the standard setup and protocol, using surface acoustic waves (SAWs) to enhance performance. Each step of the assay, from the initial antibody binding onto the walls of the well plate to the target analyte molecules' binding for detection─except, notably, for the blocking step─is improved principally via acoustic streaming-driven advection. Using SAWs, the time required for one step of an example ELISA is reduced from 60 to 15 min to achieve the same binding amount. By extending the duration of SAW exposure to 20 min, the sensitivity can be significantly improved over the 60 min, 35 °C ELISA without SAWs. It is also possible to confer beneficial improvements to bead-based ELISA by combining it with SAWs to further reduce the time required for binding to 2 min. By significantly increasing the speed of ELISA, its utility may be improved for a wide range of point-of-care diagnostics applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Adaptive Focused Acoustics (AFA) Improves the Performance of Microtiter Plate ELISAs.J Biomol Screen. 2014 Aug;19(7):1124-30. doi: 10.1177/1087057114523650. Epub 2014 Feb 19. J Biomol Screen. 2014. PMID: 24554443
-
Acoustic micromixing increases antibody-antigen binding in immunoassays.Biomed Microdevices. 2015 Aug;17(4):79. doi: 10.1007/s10544-015-9987-0. Biomed Microdevices. 2015. PMID: 26162483
-
Acoustofluidic enzyme-linked immunosorbent assay (ELISA) platform enabled by coupled acoustic streaming.Anal Chim Acta. 2019 Nov 4;1079:129-138. doi: 10.1016/j.aca.2019.05.073. Epub 2019 Jun 1. Anal Chim Acta. 2019. PMID: 31387703
-
Enzyme-Linked Immunosorbent Assay: Types and Applications.Methods Mol Biol. 2023;2612:1-17. doi: 10.1007/978-1-0716-2903-1_1. Methods Mol Biol. 2023. PMID: 36795355 Review.
-
Surface acoustic wave manipulation of bioparticles.Soft Matter. 2023 Jun 14;19(23):4166-4187. doi: 10.1039/d3sm00457k. Soft Matter. 2023. PMID: 37212436 Review.
Cited by
-
Extracellular vesicle-based point-of-care testing for diagnosis and monitoring of Alzheimer's disease.Microsyst Nanoeng. 2025 Apr 17;11(1):65. doi: 10.1038/s41378-025-00916-4. Microsyst Nanoeng. 2025. PMID: 40246821 Free PMC article.
-
Acoustofluidics: Technology Advances and Applications from 2022 to 2024.Anal Chem. 2025 Apr 8;97(13):6847-6870. doi: 10.1021/acs.analchem.4c06803. Epub 2025 Mar 25. Anal Chem. 2025. PMID: 40133046 Free PMC article. Review. No abstract available.
-
Enhancing ELISA Sensitivity: From Surface Engineering to Synthetic Biology.Biosensors (Basel). 2025 Jul 6;15(7):434. doi: 10.3390/bios15070434. Biosensors (Basel). 2025. PMID: 40710084 Free PMC article. Review.
References
-
- Udenfriend S.Fluorescence assay in biology and medicine; Academic Press: London UK, 2014; Vol. 2.
-
- Zhang Q.; Lian M.; Liu L.; Cui H. High-performance liquid chromatographic assay of parabens in wash-off cosmetic products and foods using chemiluminescence detection. Anal. Chim. Acta 2005, 537, 31–39. 10.1016/j.aca.2005.01.027. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

