Radiomics-based prediction of local control in patients with brain metastases following postoperative stereotactic radiotherapy
- PMID: 38813990
- PMCID: PMC11376458
- DOI: 10.1093/neuonc/noae098
Radiomics-based prediction of local control in patients with brain metastases following postoperative stereotactic radiotherapy
Abstract
Background: Surgical resection is the standard of care for patients with large or symptomatic brain metastases (BMs). Despite improved local control after adjuvant stereotactic radiotherapy, the risk of local failure (LF) persists. Therefore, we aimed to develop and externally validate a pre-therapeutic radiomics-based prediction tool to identify patients at high LF risk.
Methods: Data were collected from A Multicenter Analysis of Stereotactic Radiotherapy to the Resection Cavity of BMs (AURORA) retrospective study (training cohort: 253 patients from 2 centers; external test cohort: 99 patients from 5 centers). Radiomic features were extracted from the contrast-enhancing BM (T1-CE MRI sequence) and the surrounding edema (T2-FLAIR sequence). Different combinations of radiomic and clinical features were compared. The final models were trained on the entire training cohort with the best parameter set previously determined by internal 5-fold cross-validation and tested on the external test set.
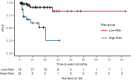
Results: The best performance in the external test was achieved by an elastic net regression model trained with a combination of radiomic and clinical features with a concordance index (CI) of 0.77, outperforming any clinical model (best CI: 0.70). The model effectively stratified patients by LF risk in a Kaplan-Meier analysis (P < .001) and demonstrated an incremental net clinical benefit. At 24 months, we found LF in 9% and 74% of the low and high-risk groups, respectively.
Conclusions: A combination of clinical and radiomic features predicted freedom from LF better than any clinical feature set alone. Patients at high risk for LF may benefit from stricter follow-up routines or intensified therapy.
Keywords: artificial intelligence; brain metastases; local failure prediction; machine learning; radiomics.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
T.B.B.: Honoraria: Merck, Takeda, Dalichi Sankyo.
A.W.: Grants: EFRE, Siemens; Consulting fees: Gilead, Hologic Medicor GmbH; Honoraria: Accuracy, Universitätsklinikum Leipzig AöR, Sanofi-Aventis GmbH; Travel support: DKFZ, DEGRO; Board: IKF GmbH (Krankenhaus Nordwest).
Cl.Z.: Co-editor on the advisory board of “Clinical Neuroradiology,” Leadership: President of the German society of Neuroradiology (DGNR)
Be. M.: Grants: BrainLab, Zeiss, Ulrich, Spineart; Royalities: Medacta, Spineart; Consulting fees and Honoraria: Medacta, Brainlab, Zeiss; Travel support: Brainlab, Medacta; Stock: Sonovum.
M.G.: Grants: Varian/Siemens Healthineers, AstraZeneca, ViewRay Inc.; Honoraria: AstraZeneca; Leadership: ESTRO president elect, SAMO board member.
N.A.: Grants: ViewRay Inc., AstraZeneca, SNF, SKL, University CRPP; Consulting Fees: ViewRay Inc., AstraZeneca; Honoraria: ViewRay Inc., AstraZeneca; Travel support: ViewRay Inc., AstraZeneca; Safety monitoring/advisory board: AstraZeneca, Equipment: ViewRay Inc.
R.A.E.S.: Grants: Accuray; Consulting Fees: Novocure, Merck, AstraZeneca; Honoraria: Accuray, AstraZeneca, BMS, Novocure, Merck, Takeda; Travel support: Merck, Accuray, AstraZeneca; Safety monitoring/advisory board: Novocure, Merck; Stock: Novocure.
J.D.: Grants: RaySearch Laboratories AB, Vision RT Limited, Merck Serono GmbH, Siemens Healthcare GmbH, PTW-Freiburg Dr. Pychlau GmbH, Accuray Incorporated; Leadership: CEO at HIT, Board of directors at University Hospital Heidelberg; Equipment: IntraOP.
O.B.: Grants: STOPSTORM.eu; Leadership: Board member of the working groups for Stereotactic Radiotherapy of the German Radiation Oncology and Medical Physics Societies, Section Editor of “Strahlentherapie und Onkologie.”
K.F.: Grants: Master of Disaster (Gyn Congress, Essen, Germany).
S.E.C.: Grants, Consulting fees and Honoraria: Roche, AstraZeneca, Medac, Dr. Sennewald Medizintechnik, Elekta, Accuray, B.M.S., Brainlab, Daiichi Sankyo, Icotec AG, Carl Zeiss Meditec AG, HMG Systems Engineering, Janssen; Safety monitoring/advisory board: CureVac DSMB Member; Leadership: NOA Board Member, DEGRO Board Member.
D.R.: Grants: DFG, ERC, EPSRC, BMBF, Alexander von Humboldt Stiftung; Consulting fees: ERC.
B.W.: Grants: DFG, NIH, Deutsche Krebshilfe, BMWi; Consulting fees and Stock: Need; Honoraria: Philips, Novartis.
J.C.P.: Honoraria: AstraZeneca, Support for current manuscript: German Research Foundation. The remaining authors have no potential conflicts of interest to disclose.
Figures

References
-
- Johnson JD, Young B.. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7(3):337–344. - PubMed
-
- Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40(5):492–516. - PubMed
-
- Buchner JA, Kofler F, Etzel L, et al. Development and external validation of an MRI-based neural network for brain metastasis segmentation in the AURORA multicenter study. Radiother Oncol. 2023;178:109425. - PubMed
-
- Buchner JA, Peeken JC, Etzel L, et al. Identifying core MRI sequences for reliable automatic brain metastasis segmentation. Radiother Oncol. 2023;188:109901. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

