Anilino-1,4-naphthoquinones as potent mushroom tyrosinase inhibitors: in vitro and in silico studies
- PMID: 38814149
- PMCID: PMC11141316
- DOI: 10.1080/14756366.2024.2357174
Anilino-1,4-naphthoquinones as potent mushroom tyrosinase inhibitors: in vitro and in silico studies
Abstract
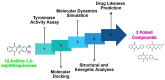
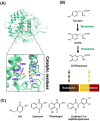
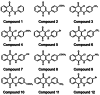
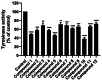
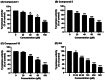
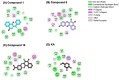
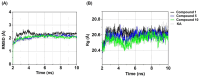
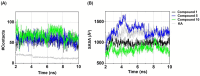
Tyrosinase, a pivotal enzyme in melanin synthesis, is a primary target for the development of depigmenting agents. In this work, in vitro and in silico techniques were employed to identify novel tyrosinase inhibitors from a set of 12 anilino-1,4-naphthoquinone derivatives. Results from the mushroom tyrosinase activity assay indicated that, among the 12 derivatives, three compounds (1, 5, and 10) demonstrated the most significant inhibitory activity against mushroom tyrosinase, surpassing the effectiveness of the kojic acid. Molecular docking revealed that all studied derivatives interacted with copper ions and amino acid residues at the enzyme active site. Molecular dynamics simulations provided insights into the stability of enzyme-inhibitor complexes, in which compounds 1, 5, and particularly 10 displayed greater stability, atomic contacts, and structural compactness than kojic acid. Drug likeness prediction further strengthens the potential of anilino-1,4-naphthoquinones as promising candidates for the development of novel tyrosinase inhibitors for the treatment of hyperpigmentation disorders.
Keywords: Tyrosinase inhibition; anilino-14-naphthoquinones; molecular docking; molecular dynamics simulations.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Ismaya WT, Rozeboom HJ, Weijn A, Mes JJ, Fusetti F, Wichers HJ, Dijkstra BW.. Crystal structure of Agaricus bisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochemistry. 2011;50(24):5477–5486. - PubMed
-
- Sansinenea E, Ortiz A.. Melanin: a photoprotection for Bacillus thuringiensis based biopesticides. Biotechnol Lett. 2015;37(3):483–490. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
