Utility of time in tight range (TITR) in evaluating metabolic control in pediatric and adult patients with type 1 diabetes in treatment with advanced hybrid closed-loop systems
- PMID: 38814372
- PMCID: PMC11489309
- DOI: 10.1007/s12020-024-03881-6
Utility of time in tight range (TITR) in evaluating metabolic control in pediatric and adult patients with type 1 diabetes in treatment with advanced hybrid closed-loop systems
Abstract
Purpose: To analyze the time in tight range (TITR), and its relationship with other glucometric parameters in patients with type 1 diabetes (T1D) treated with advanced hybrid closed-loop (AHCL) systems.
Methods: A prospective observational study was conducted on pediatric and adult patients with T1D undergoing treatment with AHCL systems for at least 3 months. Clinical variables and glucometric parameters before and after AHCL initiation were collected.
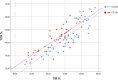
Results: A total of 117 patients were evaluated. Comparison of metabolic control after AHCL initiation showed significant improvements in HbA1c (6.9 ± 0.9 vs. 6.6 ± 0.5%, p < 0.001), time in range (TIR) (68.2 ± 11.5 vs. 82.5 ± 6.9%, p < 0.001), TITR (43.7 ± 10.8 vs. 57.3 ± 9.7%, p < 0.001), glucose management indicator (GMI) (6.9 ± 0.4 vs. 6.6 ± 0.3%, p < 0.001), time below range (TBR) 70-54 mg/dl (4.3 ± 4.5 vs. 2.0 ± 1.4%, p < 0.001), and time above range (TAR) > 180 mg/dl (36.0 ± 7.6 vs. 15.1 ± 6.4%, p < 0.001). Coefficient of variation (CV) also improved (36.3 ± 5.7 vs. 30.6 ± 3.7, p < 0.001), while time between 140-180 mg/dl remained unchanged. In total, 76.3% achieved TITR > 50% (100% pediatric). Correlation analysis between TITR and TIR and GRI showed a strong positive correlation, modified by glycemic variability.
Conclusions: AHCL systems achieve significant improvements in metabolic control (TIR > 70% in 93.9% patients). The increase in TIR was not related to an increase in TIR 140-180 mg/dl. Despite being closely related to TIR, TITR allows for a more adequate discrimination of the achieved control level, especially in a population with good initial metabolic control. The correlation between TIR and TITR is directly influenced by the degree of glycemic variability.
Keywords: Advanced hybrid closed loop; Glucose variability; Time in range; Time in tight range; Type 1 diabetes.
© 2024. The Author(s).
Conflict of interest statement
M.P.B.-C. has received speaker and consultant honoraria from Medtronic, NovoNordisk, Abbot, Lilly. G.D.-S. has received speaker and consultant honoraria from Medtronic, NovoNordisk, Abbot, Lilly. The other authors have no relevant financial or non-financial interests to disclose. PBC and PFV contributed equally to this study and share first authorship.
Figures
References
-
- M. Yaron, E. Roitman, G. Aharon-Hananel, Z. Landau, T. Ganz, I. Yanuv, A. Rozenberg, M. Karp, M. Ish-Shalom, J. Singer, J. Wainstein, I. Raz, Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care 42(7), 1178–1184 (2019). 10.2337/dc18-0166 - DOI - PubMed
-
- T. Danne, R. Nimri, T. Battelino, R.M. Bergenstal, K.L. Close, J.H. DeVries, S. Garg, L. Heinemann, I. Hirsch, S.A. Amiel, R. Beck, E. Bosi, B. Buckingham, C. Cobelli, E. Dassau, F.J. Doyle 3rd, S. Heller, R. Hovorka, W. Jia, T. Jones, O. Kordonouri, B. Kovatchev, A. Kowalski, L. Laffel, D. Maahs, H.R. Murphy, K. Nørgaard, C.G. Parkin, E. Renard, B. Saboo, M. Scharf, W.V. Tamborlane, S.A. Weinzimer, M. Phillip, International Consensus on use of continuous glucose monitoring. Diabetes Care 40(12), 1631–1640 (2017). 10.2337/dc17-1600 - DOI - PMC - PubMed
-
- T. Battelino, T. Danne, R.M. Bergenstal, S.A. Amiel, R. Beck, T. Biester, E. Bosi, B.A. Buckingham, W.T. Cefalu, K.L. Close, C. Cobelli, E. Dassau, J.H. DeVries, K.C. Donaghue, K. Dovc, F.J. Doyle 3rd, S. Garg, G. Grunberger, S. Heller, L. Heinemann, I.B. Hirsch, R. Hovorka, W. Jia, O. Kordonouri, B. Kovatchev, A. Kowalski, L. Laffel, B. Levine, A. Mayorov, C. Mathieu, H.R. Murphy, R. Nimri, K. Nørgaard, C.G. Parkin, E. Renard, D. Rodbard, B. Saboo, D. Schatz, K. Stoner, T. Urakami, S.A. Weinzimer, M. Phillip, Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on time in range. Diabetes Care 42(8), 1593–1603 (2019). 10.2337/dci19-0028 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical