Application of Physiologically Based Pharmacokinetic Modeling to Characterize the Effects of Age and Obesity on the Disposition of Levetiracetam in the Pediatric Population
- PMID: 38814425
- PMCID: PMC11225543
- DOI: 10.1007/s40262-024-01367-2
Application of Physiologically Based Pharmacokinetic Modeling to Characterize the Effects of Age and Obesity on the Disposition of Levetiracetam in the Pediatric Population
Abstract
Background: Levetiracetam is an antiseizure medication used for several seizure types in adults and children aged 1 month and older; however, due to a lack of data, pharmacokinetic (PK) variability of levetiracetam is not adequately characterized in certain populations, particularly neonates, children younger than 2 years of age, and children older than 2 years of age with obesity.
Objective: This study aimed to address the gap by leveraging PK data from two prospective standard-of-care pediatric trials (n = 88) covering an age range from 1 month to 19 years, including those with obesity (64%), and applying a physiologically based PK (PBPK) modeling framework.
Methods: A published PBPK model of levetiracetam for children aged 2 years and older was extended to pediatric patients younger than 2 years of age and patients older than 2 years of age with obesity by accounting for the obesity and age-related changes in PK using PK-Sim® software. The prospective pediatric data, along with the literature data for neonates and children younger than 2 years of age, were used to evaluate the extended PBPK models.
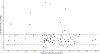
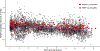
Results: Overall, 82.4% of data fell within the 90% interval of model-predicted concentrations, with an average fold error within twofold of the accepted criteria. PBPK modeling revealed that children with obesity had lower weight-normalized clearances (0.053 L/h/kg) on average than children without obesity (0.063 L/h/kg). The effect of maturation was well-characterized, resulting in comparable PBPK-simulated, weight-normalized clearances for neonates and children younger than 2 years of age reported from the literature.
Conclusions: PBPK modeling simulations revealed that the current US FDA-labeled pediatric dosing regimen listed in the prescribing information can produce the required exposure of levetiracetam in these target populations with dose adjustments for children with obesity aged 4 years to younger than 16 years.
© 2024. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Figures
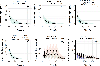

References
-
- Levetiracetam Actavis Oral Solution [Internet]. [cited 2024 Feb 1]. Available from: https://www.ema.europa.eu/en/documents/product-information/levetiracetam.... Accessed 1 Feb 2024.
-
- Keppra Package Insert [Internet]. [cited 2022 Dec 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021035s099,021.... Accessed 8 Dec 2022.
-
- Off-Label Use of Antiepileptic Drugs for the Treatment of Neonatal Seizures - ScienceDirect [Internet]. [cited 2023 Feb 2]. Available from: https://www-sciencedirect-com.libproxy.lib.unc.edu/science/article/pii/S.... Accessed 2 Feb 2023.
-
- Patsalos PN. Clinical Pharmacokinetics of Levetiracetam: Clin Pharmacokinet. 2004;43:707–24. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

