Population pharmacokinetic, pharmacodynamic and efficacy modeling of SB12 (proposed eculizumab biosimilar) and reference eculizumab
- PMID: 38814441
- PMCID: PMC11303580
- DOI: 10.1007/s00228-024-03703-8
Population pharmacokinetic, pharmacodynamic and efficacy modeling of SB12 (proposed eculizumab biosimilar) and reference eculizumab
Abstract
Purpose: To describe, compare similarity of pharmacokinetic (PK), pharmacodynamic (PD) and efficacy of SB12 and reference eculizumab (ECU) and find clinically significant covariate relationships.
Methods: The PK, PD (terminal complement activity) and efficacy (LDH) data of SB12 and ECU were obtained from 289 subjects from phase I and phase III studies. One- and two-compartment PK models with first-order elimination were evaluated for SB12 and ECU. For PD and efficacy, both direct and indirect models were tested. The impact of covariates on PK, PD and efficacy parameters was assessed. Relationship between PK/PD and PD/efficacy was characterized. This modeling was performed using NONMEM version 7.4 (Icon Development Solutions, Ellicott City, MD, USA).
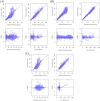
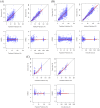
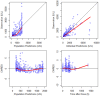
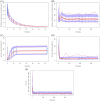
Results: The two-compartment model adequately described the PK of SB12 and ECU, and the subject's weight was chosen as a clinically significant covariate affecting drugs' clearance and central volume of distribution. Treatment group was not a significant covariate affecting clearance. The direct response model using inhibitory sigmoid Emax and sigmoid Emax relationship well described the PK/PD relationship and PD/efficacy relationship of SB12 and ECU, respectively. Through this modeling, the relationships between PK, PD and efficacy were characterized. There were no differences in PK, PD and efficacy parameters between SB12 and ECU in pooled populations of healthy subjects and paroxysmal nocturnal haemoglobinuria (PNH) patients.
Conclusion: The population modeling showed PK, PD and efficacy similarities between SB12 and ECU in pooled population of healthy subjects and PNH patients, supporting the totality of evidence on biosimilarity for SB12.
Keywords: Biosimilar; Eculizumab; Paroxysmal nocturnal hemoglobinuria; Population modeling; SB12.
© 2024. The Author(s).
Conflict of interest statement
All authors are employees of either Samsung Bioepis Co., Ltd. or of an organization contracted by Samsung Bioepis Co., Ltd. for the present study. There are no other relationships or activities that could appear to influence the submitted work
Figures
Similar articles
-
A phase III, randomised, double-blind, multi-national clinical trial comparing SB12 (proposed eculizumab biosimilar) and reference eculizumab in patients with paroxysmal nocturnal haemoglobinuria.EJHaem. 2022 Dec 20;4(1):26-36. doi: 10.1002/jha2.632. eCollection 2023 Feb. EJHaem. 2022. PMID: 36819188 Free PMC article.
-
EPYSQLI (SB12; Biosimilar to Reference Eculizumab) in Asian and Non-Asian Patients With Paroxysmal Nocturnal Hemoglobinuria: Subgroup Analysis of a Global Phase III Randomized Controlled Trial.EJHaem. 2025 Mar 21;6(2):e70020. doi: 10.1002/jha2.70020. eCollection 2025 Apr. EJHaem. 2025. PMID: 40123793 Free PMC article.
-
Characterization for the Similarity Assessment between Proposed Biosimilar SB12 and Eculizumab Reference Product Using a State-of-the-Art Analytical Method.BioDrugs. 2023 Jul;37(4):569-581. doi: 10.1007/s40259-023-00591-9. Epub 2023 Apr 15. BioDrugs. 2023. PMID: 37060541 Free PMC article.
-
Eculizumab: a review of its use in paroxysmal nocturnal haemoglobinuria.Drugs. 2011 Dec 3;71(17):2327-45. doi: 10.2165/11208300-000000000-00000. Drugs. 2011. PMID: 22085388 Review.
-
Anti-complement Treatment for Paroxysmal Nocturnal Hemoglobinuria: Time for Proximal Complement Inhibition? A Position Paper From the SAAWP of the EBMT.Front Immunol. 2019 Jun 14;10:1157. doi: 10.3389/fimmu.2019.01157. eCollection 2019. Front Immunol. 2019. PMID: 31258525 Free PMC article. Review.
Cited by
-
Does one model fit all mAbs? An evaluation of population pharmacokinetic models.MAbs. 2025 Dec;17(1):2512217. doi: 10.1080/19420862.2025.2512217. Epub 2025 May 30. MAbs. 2025. PMID: 40447562 Free PMC article.
-
Complement-targeted therapeutics: Are we there yet, or just getting started?Eur J Immunol. 2024 Dec;54(12):e2350816. doi: 10.1002/eji.202350816. Epub 2024 Sep 12. Eur J Immunol. 2024. PMID: 39263829 Free PMC article. Review.
References
-
- Shah N, Bhatt H (2023) Paroxysmal nocturnal hemoglobinuria [Book] StatPearls Publishing - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

