Activation of the hypoxia-inducible factor pathway by roxadustat improves glucose metabolism in human primary myotubes from men
- PMID: 38814443
- PMCID: PMC11410918
- DOI: 10.1007/s00125-024-06185-6
Activation of the hypoxia-inducible factor pathway by roxadustat improves glucose metabolism in human primary myotubes from men
Abstract
Aims/hypothesis: Hypoxia-inducible factor prolyl 4-hydroxylase (HIF-P4H) enzymes regulate adaptive cellular responses to low oxygen concentrations. Inhibition of HIF-P4Hs leads to stabilisation of hypoxia-inducible factors (HIFs) and activation of the HIF pathway affecting multiple biological processes to rescue cells from hypoxia. As evidence from animal models suggests that HIF-P4H inhibitors could be used to treat metabolic disorders associated with insulin resistance, we examined whether roxadustat, an HIF-P4H inhibitor approved for the treatment of renal anaemia, would have an effect on glucose metabolism in primary human myotubes.
Methods: Primary skeletal muscle cell cultures, established from biopsies of vastus lateralis muscle from men with normal glucose tolerance (NGT) (n=5) or type 2 diabetes (n=8), were treated with roxadustat. Induction of HIF target gene expression was detected with quantitative real-time PCR. Glucose uptake and glycogen synthesis were investigated with radioactive tracers. Glycolysis and mitochondrial respiration rates were measured with a Seahorse analyser.
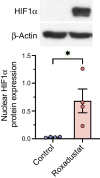
Results: Exposure to roxadustat stabilised nuclear HIF1α protein expression in human myotubes. Treatment with roxadustat led to induction of HIF target gene mRNAs for GLUT1 (also known as SLC2A1), HK2, MCT4 (also known as SLC16A4) and HIF-P4H-2 (also known as PHD2 or EGLN1) in myotubes from donors with NGT, with a blunted response in myotubes from donors with type 2 diabetes. mRNAs for LDHA, PDK1 and GBE1 were induced to a similar degree in myotubes from donors with NGT or type 2 diabetes. Exposure of myotubes to roxadustat led to a 1.4-fold increase in glycolytic rate in myotubes from men with NGT (p=0.0370) and a 1.7-fold increase in myotubes from donors with type 2 diabetes (p=0.0044), with no difference between the groups (p=0.1391). Exposure to roxadustat led to a reduction in basal mitochondrial respiration in both groups (p<0.01). Basal glucose uptake rates were similar in myotubes from donors with NGT (20.2 ± 2.7 pmol mg-1 min-1) and type 2 diabetes (25.3 ± 4.4 pmol mg-1 min-1, p=0.4205). Treatment with roxadustat enhanced insulin-stimulated glucose uptake in myotubes from donors with NGT (1.4-fold vs insulin-only condition, p=0.0023). The basal rate of glucose incorporation into glycogen was lower in myotubes from donors with NGT (233 ± 12.4 nmol g-1 h-1) than in myotubes from donors with type 2 diabetes (360 ± 40.3 nmol g-1 h-1, p=0.0344). Insulin increased glycogen synthesis by 1.9-fold (p=0.0025) in myotubes from donors with NGT, whereas roxadustat did not affect their basal or insulin-stimulated glycogen synthesis. Insulin increased glycogen synthesis by 1.7-fold (p=0.0031) in myotubes from donors with type 2 diabetes. While basal glycogen synthesis was unaffected by roxadustat, pretreatment with roxadustat enhanced insulin-stimulated glycogen synthesis in myotubes from donors with type 2 diabetes (p=0.0345).
Conclusions/interpretation: Roxadustat increases glycolysis and inhibits mitochondrial respiration in primary human myotubes regardless of diabetes status. Roxadustat may also improve insulin action on glycogen synthesis in myotubes from donors with type 2 diabetes.
Keywords: Glucose metabolism; Hypoxia-inducible factor; Insulin resistance; Insulin signalling; Primary human muscle cells; Roxadustat.
© 2024. The Author(s).
Figures


Similar articles
-
Activation of the hypoxia-inducible factor pathway protects against acute ischemic stroke by reprogramming central carbon metabolism.Theranostics. 2024 Apr 29;14(7):2856-2880. doi: 10.7150/thno.88223. eCollection 2024. Theranostics. 2024. PMID: 38773968 Free PMC article.
-
Nonclinical Characterization of the Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat, a Novel Treatment of Anemia of Chronic Kidney Disease.J Pharmacol Exp Ther. 2020 Aug;374(2):342-353. doi: 10.1124/jpet.120.265181. Epub 2020 Jun 2. J Pharmacol Exp Ther. 2020. PMID: 32487538
-
Hypoxia-Inducible Factor Prolyl 4-Hydroxylase-2 Inhibition Protects Against Development of Atherosclerosis.Arterioscler Thromb Vasc Biol. 2016 Apr;36(4):608-17. doi: 10.1161/ATVBAHA.115.307136. Epub 2016 Feb 4. Arterioscler Thromb Vasc Biol. 2016. PMID: 26848160
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
-
Hypoxia-Inducible Factor Prolyl 4-Hydroxylases and Metabolism.Trends Mol Med. 2018 Dec;24(12):1021-1035. doi: 10.1016/j.molmed.2018.10.004. Epub 2018 Nov 1. Trends Mol Med. 2018. PMID: 30391126 Review.
Cited by
-
Targeting HIF-P4H-2 in APP/PS1 Alzheimer's mouse model improves glucose metabolism, reduces dystrophic neuritis, and maintains exploratory activity.J Biol Chem. 2025 Jul 1;301(8):110432. doi: 10.1016/j.jbc.2025.110432. Online ahead of print. J Biol Chem. 2025. PMID: 40609789 Free PMC article.
-
REDOX Imbalance and Oxidative Stress in the Intervertebral Disc: The Effect of Mechanical Stress and Cigarette Smoking on ER Stress and Mitochondrial Dysfunction.Cells. 2025 Apr 19;14(8):613. doi: 10.3390/cells14080613. Cells. 2025. PMID: 40277939 Free PMC article. Review.
References
-
- Semenza GL (2020) The genomics and genetics of oxygen homeostasis. Annu Rev Genomics Hum Genet 21(1):183–204. 10.1146/annurev-genom-111119-073356 - PubMed
-
- Weidemann A, Johnson RS (2008) Biology of HIF-1alpha. Cell Death Differ 15(4):621–627. 10.1038/cdd.2008.12 - PubMed
-
- Kierans SJ, Taylor CT (2021) Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol 599(1):23–37. 10.1113/JP280572 - PubMed
-
- Wang GL, Semenza GL (1995) Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 270(3):1230–1237. 10.1074/jbc.270.3.1230 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

