Risk Factors and Clinical Significance of Ultra-Long-Term Microischemia After Intracranial Aneurysm Embolization
- PMID: 38814531
- PMCID: PMC11263440
- DOI: 10.1007/s40120-024-00630-9
Risk Factors and Clinical Significance of Ultra-Long-Term Microischemia After Intracranial Aneurysm Embolization
Abstract
Introduction: This study aimed to explore influencing factors and clinical significance of ultra-long-term microischemia following intracranial aneurysm (IA) embolization and establish a theoretical foundation for reducing both the incidence of ultra-long-term microischemia and cognitive dysfunction in patients post embolization.
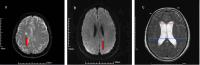
Methods: A retrospective analysis was conducted on data from 147 patients who received endovascular treatment for IAs. Patients were categorized into microischemic and control (non-microischemic) groups on the based on the findings of high-resolution magnetic resonance vessel wall imaging (HR-VWI) examinations performed 3 days postoperatively and 6 months postoperatively. Risk factors for the occurrence of ultra-long-term microischemia were determined by univariate analysis and multivariate logistic regression analysis.
Results: Out of 147 patients included in the study, 51 (34.69%) developed microischemia while the remaining 96 (65.31%) did not experience this condition. Analysis revealed that factors such as sex, age, history of underlying diseases (hypertension, diabetes mellitus), aneurysmal site characteristics, the presence or absence of stenosis in the aneurysm-bearing artery, modified Fisher score at admission, Barthel's index at discharge, immunoinflammatory index at 3 days postoperatively and at the 6-month follow-up, the presence or absence of aneurysmal wall enhancement, and the presence or absence of aneurysmal lumen showed no statistically significant differences between the two groups (all P > 0.05). By contrast, variables like in operative time, rupture status of the aneurysm before surgery according to World Federation of Neurologic Surgeons (WFNS) grade, aneurysm size, number of stents used, number of guidewires and catheters used, and Evans index between the two groups were found to have statistically significant disparities between those who developed microischemia and those who did not (P < 0.05). A subsequent multivariate analysis revealed that aneurysm size, Evans index, and the number of stents used were independent risk factors for the occurrence of ultra-long-term microischemia after surgical intervention of aneurysms (P < 0.05). The receiver operating characteristic (ROC) curves of the patients were constructed on the basis of risk factors determined through multivariate logistic regression analysis. Results indicated that aneurysm size (area under ROC curve (AUC) 0.619, sensitivity 94.7%, specificity 17.1%, P = 0.049), Evans index (AUC 0.670, sensitivity 96.4%, specificity 26.8%, P = 0.004), and number of stents (AUC 0.639, sensitivity 44.6%, specificity 90.2%, P < 0.001) effectively predicted the occurrence of microischemia. The incidence of cognitive dysfunction was higher in the microischemic group than in the control group (P < 0.05), and a greater number of microischemic foci was associated with a higher incidence of cognitive dysfunction. The proportion of microschemia foci in the thalamus and basal ganglia in patients with cognitive dysfunction (60.87%) was significantly higher than that in patients without cognitive dysfunction (34.55%) (P < 0.05).
Conclusion: Aneurysm size, Evans index > 0.3, and the quantity of stents were independent risk factors for the occurrence of ultra-long-term microischemia after aneurysm embolization and provided good predictive performance. Cognitive dysfunction was closely associated with microischemia, with its severity increasing with an increase in the number of ischemic foci.
Keywords: Embolization; Intracranial aneurysm; Microischemia; Risk factors.
© 2024. The Author(s).
Conflict of interest statement
Yi Song, Jianxin Zhou, Yun Tan, Yao Wu, Mingdong Liu and Yuan Cheng certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures


References
-
- Kane SF, Butler E, Sindelar BD. Nontraumatic subarachnoid hemorrhage and ruptured intracranial aneurysm: recognition and evaluation. Am Fam Phys. 2023;108(4):386–95. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

