Vascular Endothelial Growth Factor Receptor Inhibitors for Recurrent or Metastatic Adenoid Cystic Carcinoma: A Systematic Review and Meta-Analysis
- PMID: 38814585
- PMCID: PMC11140580
- DOI: 10.1001/jamaoto.2024.1177
Vascular Endothelial Growth Factor Receptor Inhibitors for Recurrent or Metastatic Adenoid Cystic Carcinoma: A Systematic Review and Meta-Analysis
Abstract
Importance: There is no systemic therapy for recurrent or metastatic adenoid cystic carcinoma (ACC) approved by the US Food and Drug Administration.
Objective: To examine the efficacy, safety, and tolerability of vascular endothelial growth factor receptor (VEGFR) inhibitors in recurrent or metastatic ACC.
Data sources: PubMed, Embase, and Cochrane Library were systematically searched for studies of VEGFR inhibitors in recurrent or metastatic ACC from database inception to August 31, 2023.
Study selection: Inclusion criteria were prospective clinical trials of recurrent or metastatic ACC treated with VEGFR inhibitors, reporting at least 1 outcome of interest specifically for ACC. Of 1963 identified studies, 17 (0.9%) met inclusion criteria.
Data extraction and synthesis: The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) reporting guideline was followed to extract data. Data were pooled using a random-effects generalized linear mixed model with 95% CIs.
Main outcomes and measures: The primary efficacy outcome was best overall response to VEGFR inhibitors, including objective response, stable disease, or progressive disease (PD). Safety and tolerability outcomes included incidence of grade 3 or higher adverse events, rates of exit from trial due to PD or drug-related toxic effects, and dose reduction rate (DRR).
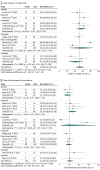
Results: A total of 17 studies comprising 560 patients with recurrent or metastatic ACC treated with 10 VEGFR inhibitors were included. The objective response rate was 6% (95% CI, 3%-12%; I2 = 71%) and stable disease was the most frequent best overall response (82%; 95% CI, 74%-87%; I2 = 67%). The 6-month disease control (defined as objective response and stable disease) rate was 54% (95% CI, 45%-62%; I2 = 52%). The rate of grade 3 or higher adverse events was 53% (95% CI, 42%-64%; I2 = 81%) and of DRR was 59% (95% CI, 40%-76%). Most patients (57%; 95% CI, 44%-70%; I2 = 83%) continued therapy until PD; 21% (95% CI, 15%-28%; I2 = 62%) of patients suspended therapy for toxic effects. In subgroup analysis by specific VEGFR inhibitor, the objective response rate was 14% (95% CI, 7%-25%; I2 = 0%), stable disease rate was 76% (95% CI, 63%-85%; I2 = 0%), proportion treated until PD was 61% (95% CI, 14%-94%; I2 = 94%), and DRR was 78% (95% CI, 66%-87%; I2 = 39%) with lenvatinib. Corresponding axitinib results were objective response rate of 8% (95% CI, 4%-15%; I2 = 0%) and stable disease rate of 85% (95% CI, 72%-92%; I2 = 69%), with 73% (95% CI, 63%-82%; I2 = 0%) of patients treated until PD, and the DRR was 22% (95% CI, 12%-38%; I2 = 77%). Rivoceranib had the highest objective response rate (24%; 95% CI, 7%-57%) but high heterogeneity among studies (I2 = 95%) and the lowest rate of patients who continued therapy until PD (35%; 95% CI, 20%-55%; I2 = 90%).
Conclusions and relevance: This systematic review and meta-analysis found that VEGFR inhibitors were associated with high rates of disease stabilization in recurrent or metastatic ACC. Of 10 included VEGFR inhibitors, lenvatinib and axitinib were associated with the best combined and consistent efficacy, safety, and tolerability profiles, substantiating their inclusion in treatment guidelines.
Conflict of interest statement
Figures



Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
A Phase II Trial of Rivoceranib, an Oral Vascular Endothelial Growth Factor Receptor 2 Inhibitor, for Recurrent or Metastatic Adenoid Cystic Carcinoma.Clin Cancer Res. 2023 Nov 14;29(22):4555-4563. doi: 10.1158/1078-0432.CCR-23-1030. Clin Cancer Res. 2023. PMID: 37643133 Free PMC article. Clinical Trial.
-
Analysis of Response and Progression Patterns of Tyrosine Kinase Inhibitors in Recurrent or Metastatic Adenoid Cystic Carcinoma: A Post Hoc Analysis of Two KCSG Phase II Trials.Cancer Res Treat. 2024 Oct;56(4):1068-1076. doi: 10.4143/crt.2024.008. Epub 2024 Apr 15. Cancer Res Treat. 2024. PMID: 38637966 Free PMC article. Clinical Trial.
-
Phase II trial of dasatinib for recurrent or metastatic c-KIT expressing adenoid cystic carcinoma and for nonadenoid cystic malignant salivary tumors.Ann Oncol. 2016 Feb;27(2):318-23. doi: 10.1093/annonc/mdv537. Epub 2015 Nov 23. Ann Oncol. 2016. PMID: 26598548 Free PMC article. Clinical Trial.
-
The effectiveness of targeted therapy for recurrence or metastasis adenoid cystic carcinoma: a systematic review and meta-analysis.Ann Med. 2024 Dec;56(1):2399867. doi: 10.1080/07853890.2024.2399867. Epub 2024 Sep 11. Ann Med. 2024. PMID: 39258959 Free PMC article.
Cited by
-
Clinical Outcomes With Notch Inhibitors in Notch-Activated Recurrent/Metastatic Adenoid Cystic Carcinoma.Cancer Med. 2025 Mar;14(5):e70663. doi: 10.1002/cam4.70663. Cancer Med. 2025. PMID: 40025676 Free PMC article.
-
Comparison of postoperative radiotherapy and definitive radiotherapy for non-metastatic adenoid cystic carcinoma of the head and neck, a propensity score matching based on the SEER database.Transl Cancer Res. 2024 Nov 30;13(11):6045-6056. doi: 10.21037/tcr-24-1221. Epub 2024 Nov 27. Transl Cancer Res. 2024. PMID: 39697727 Free PMC article.
References
-
- Pfister DG, Spencer S, Adkins D, et al. ; National Comprehensive Cancer Network . Head and Neck Cancers (Version 1.2024). Accessed November 12, 2023. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
-
- Terhaard CH, Lubsen H, Van der Tweel I, et al. ; Dutch Head and Neck Oncology Cooperative Group . Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck. 2004;26(8):681-692. doi:10.1002/hed.10400 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

