In vivo whole-cortex marker of excitation-inhibition ratio indexes cortical maturation and cognitive ability in youth
- PMID: 38814872
- PMCID: PMC11161789
- DOI: 10.1073/pnas.2318641121
In vivo whole-cortex marker of excitation-inhibition ratio indexes cortical maturation and cognitive ability in youth
Abstract
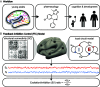
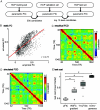
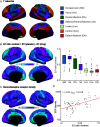
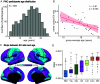
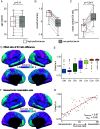
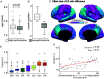
A balanced excitation-inhibition ratio (E/I ratio) is critical for healthy brain function. Normative development of cortex-wide E/I ratio remains unknown. Here, we noninvasively estimate a putative marker of whole-cortex E/I ratio by fitting a large-scale biophysically plausible circuit model to resting-state functional MRI (fMRI) data. We first confirm that our model generates realistic brain dynamics in the Human Connectome Project. Next, we show that the estimated E/I ratio marker is sensitive to the gamma-aminobutyric acid (GABA) agonist benzodiazepine alprazolam during fMRI. Alprazolam-induced E/I changes are spatially consistent with positron emission tomography measurement of benzodiazepine receptor density. We then investigate the relationship between the E/I ratio marker and neurodevelopment. We find that the E/I ratio marker declines heterogeneously across the cerebral cortex during youth, with the greatest reduction occurring in sensorimotor systems relative to association systems. Importantly, among children with the same chronological age, a lower E/I ratio marker (especially in the association cortex) is linked to better cognitive performance. This result is replicated across North American (8.2 to 23.0 y old) and Asian (7.2 to 7.9 y old) cohorts, suggesting that a more mature E/I ratio indexes improved cognition during normative development. Overall, our findings open the door to studying how disrupted E/I trajectories may lead to cognitive dysfunction in psychopathology that emerges during youth.
Keywords: cognition; control network; default mode network; neurodevelopment; resting state functional connectivity.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
Update of
-
In-vivo whole-cortex marker of excitation-inhibition ratio indexes cortical maturation and cognitive ability in youth.bioRxiv [Preprint]. 2024 Mar 28:2023.06.22.546023. doi: 10.1101/2023.06.22.546023. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Jun 4;121(23):e2318641121. doi: 10.1073/pnas.2318641121. PMID: 38586012 Free PMC article. Updated. Preprint.
References
-
- Turrigiano G. G., Nelson S. B., Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107 (2004). - PubMed
MeSH terms
Substances
Grants and funding
- R37 MH125829/MH/NIMH NIH HHS/United States
- OFLCG19May-0035; STaR20nov-0003/Singapore National Medical Research Council
- R01 MH120482/MH/NIMH NIH HHS/United States
- R00 MH127293/MH/NIMH NIH HHS/United States
- RF1 MH116920/MH/NIMH NIH HHS/United States
- R01 MH113550/MH/NIMH NIH HHS/United States
- T32 MH014654/MH/NIMH NIH HHS/United States
- CG21APR1009/Singapore Ministry of Health
- U54 MH091657/MH/NIMH NIH HHS/United States
- R01 MH133334/MH/NIMH NIH HHS/United States
- R01MH120080/HHS | National Institutes of Health (NIH)
- K99MH127293; T32MH014654/HHS | National Institutes of Health (NIH)
- K99 MH127293/MH/NIMH NIH HHS/United States
- RC2 MH089983/MH/NIMH NIH HHS/United States
- NUHSRO/2020/124/TMR/LOA/NUS Yong Loo Lin School of Medicine
- T32 MH019112/MH/NIMH NIH HHS/United States
- R01 EB022573/EB/NIBIB NIH HHS/United States
- R01 MH112847/MH/NIMH NIH HHS/United States
- RF1 MH121867/MH/NIMH NIH HHS/United States
- R01MH113550; R01MH120482; R01MH112847; R01EB022573; RF1MH116920; RF1MH121867; R37MH125829; T32MH019/HHS | National Institutes of Health (NIH)
- RC2 MH089924/MH/NIMH NIH HHS/United States
- R01 MH120080/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

