Single-exon fetal RHD genotyping: a 31-month follow up in the obstetric population of Western Sweden
- PMID: 38814881
- PMCID: PMC11390609
- DOI: 10.2450/BloodTransfus.741
Single-exon fetal RHD genotyping: a 31-month follow up in the obstetric population of Western Sweden
Abstract
Background: The Rh blood group system is highly complex, polymorphic, and immunogenic. The presence of RHD gene variants in RhD negative pregnant women is a challenge in fetal RHD genotyping as it may influence the antenatal management of anti-D prophylaxis. The aim of this study was to determine the efficiency of a non-invasive single-exon approach in the obstetric population of Western Sweden in a 31-month follow up. The frequency and type of maternal RHD variants were explored and the relation to the ethnicity was elucidated. Discrepant results between fetal RHD genotyping and serological blood group typing of newborns were investigated and clarified.
Materials and methods: RHD exon 4 was analysed with quantitative real-time PCR technique in a total of 6,948 blood samples from RhD negative women in early pregnancy. All cases with suspected maternal RHD gene and discrepant results observed in newborn samples, were further investigated using both serological and molecular technologies.
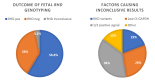
Results: A total of 43 samples (0.6%) had inconclusive fetal genotyping result due the presence of a maternal RHD gene. These findings were in most cases (>66%) observed in pregnant women of non-European ancestry. Additionally, two novel RHD alleles were found. Seven discrepant results between fetal RHD genotype and serological RhD type of the newborns, were shown to be related to D antigen variants in newborns. Assay sensitivity was 99.95%, specificity 100%, and accuracy 99.97%.
Discussion: The single-exon approach for fetal RHD screening early in pregnancy is an appropriate choice in the population of Western Sweden, with a very low frequency of inconclusive results caused by the presence of maternal RHD gene variants. Due to the high sensitivity, specificity, and accuracy of the test, serological typing of neonates born to RhD negative women has no longer been performed at our laboratory since June 2023.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures
Similar articles
-
Evaluation of an automated platform for non-invasive single-exon fetal RHD genotyping early in pregnancy.Blood Transfus. 2023 Nov 14;21(6):472-478. doi: 10.2450/2023.0267-22. Blood Transfus. 2023. PMID: 36795345 Free PMC article.
-
Determination of fetal RHD type in plasma of RhD negative pregnant women.Scand J Clin Lab Invest. 2018 Sep;78(5):411-416. doi: 10.1080/00365513.2018.1475681. Epub 2018 Jun 5. Scand J Clin Lab Invest. 2018. PMID: 29869532
-
Non-invasive fetal RHD exon 7 and exon 10 genotyping using real-time PCR testing of fetal DNA in maternal plasma.Fetal Diagn Ther. 2005 Jul-Aug;20(4):275-80. doi: 10.1159/000085085. Fetal Diagn Ther. 2005. PMID: 15980640
-
Noninvasive Fetal RhD Blood Group Genotyping: A Health Technology Assessment.Ont Health Technol Assess Ser. 2020 Nov 2;20(15):1-160. eCollection 2020. Ont Health Technol Assess Ser. 2020. PMID: 33240456 Free PMC article. Review.
-
Diagnostic accuracy of non-invasive fetal RhD genotyping using cell-free fetal DNA: a meta analysis.J Matern Fetal Neonatal Med. 2014 Dec;27(18):1839-44. doi: 10.3109/14767058.2014.882306. Epub 2014 Feb 10. J Matern Fetal Neonatal Med. 2014. PMID: 24422551 Review.
References
-
- Hemker MB, Ligthart PC, Berger L, van Rhenen DJ, van der Schoot CE, Wijk PA. DAR, a new RhD variant involving exons 4, 5, and 7, often in linkage with ceAR, a new Rhce variant frequently found in African blacks. Blood. 1999;94:4337–4342. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources