Negation mitigates rather than inverts the neural representations of adjectives
- PMID: 38814982
- PMCID: PMC11139306
- DOI: 10.1371/journal.pbio.3002622
Negation mitigates rather than inverts the neural representations of adjectives
Abstract
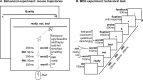
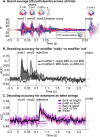
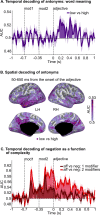
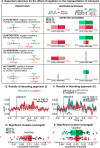
Combinatoric linguistic operations underpin human language processes, but how meaning is composed and refined in the mind of the reader is not well understood. We address this puzzle by exploiting the ubiquitous function of negation. We track the online effects of negation ("not") and intensifiers ("really") on the representation of scalar adjectives (e.g., "good") in parametrically designed behavioral and neurophysiological (MEG) experiments. The behavioral data show that participants first interpret negated adjectives as affirmative and later modify their interpretation towards, but never exactly as, the opposite meaning. Decoding analyses of neural activity further reveal significant above chance decoding accuracy for negated adjectives within 600 ms from adjective onset, suggesting that negation does not invert the representation of adjectives (i.e., "not bad" represented as "good"); furthermore, decoding accuracy for negated adjectives is found to be significantly lower than that for affirmative adjectives. Overall, these results suggest that negation mitigates rather than inverts the neural representations of adjectives. This putative suppression mechanism of negation is supported by increased synchronization of beta-band neural activity in sensorimotor areas. The analysis of negation provides a steppingstone to understand how the human brain represents changes of meaning over time.
Copyright: © 2024 Zuanazzi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Comment in
-
"Not" in the brain and behavior.PLoS Biol. 2024 May 31;22(5):e3002656. doi: 10.1371/journal.pbio.3002656. eCollection 2024 May. PLoS Biol. 2024. PMID: 38820496 Free PMC article.
References
-
- Oseki Y, Marantz A. Modeling morphological processing in human magnetoencephalography. Proceedings of the Society for Computation in Linguistics. New York, NY: Association for Computational Linguistics; 2020. pp. 431–441.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

