A novel ilarvirus protein CP-RT is expressed via stop codon readthrough and suppresses RDR6-dependent RNA silencing
- PMID: 38814986
- PMCID: PMC11166343
- DOI: 10.1371/journal.ppat.1012034
A novel ilarvirus protein CP-RT is expressed via stop codon readthrough and suppresses RDR6-dependent RNA silencing
Abstract
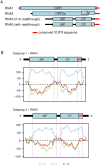
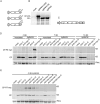
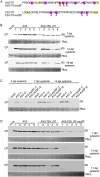
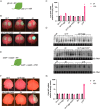
Ilarviruses are a relatively understudied but important group of plant RNA viruses that includes a number of crop pathogens. Their genomes comprise three RNA segments encoding two replicase subunits, movement protein, coat protein (CP), and (in some ilarvirus subgroups) a protein that suppresses RNA silencing. Here we report that, in many ilarviruses, RNA3 encodes an additional protein (termed CP-RT) as a result of ribosomal readthrough of the CP stop codon into a short downstream readthrough (RT) ORF. Using asparagus virus 2 as a model, we find that CP-RT is expressed in planta where it functions as a weak suppressor of RNA silencing. CP-RT expression is essential for persistent systemic infection in leaves and shoot apical meristem. CP-RT function is dependent on a putative zinc-finger motif within RT. Replacing the asparagus virus 2 RT with the RT of an ilarvirus from a different subgroup restored the ability to establish persistent infection. These findings open up a new avenue for research on ilarvirus silencing suppression, persistent meristem invasion and vertical transmission.
Copyright: © 2024 Lukhovitskaya et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
A Stem-Loop Structure in Potato Leafroll Virus Open Reading Frame 5 (ORF5) Is Essential for Readthrough Translation of the Coat Protein ORF Stop Codon 700 Bases Upstream.J Virol. 2018 May 14;92(11):e01544-17. doi: 10.1128/JVI.01544-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29514911 Free PMC article.
-
RNA silencing-related genes contribute to tolerance of infection with potato virus X and Y in a susceptible tomato plant.Virol J. 2020 Oct 8;17(1):149. doi: 10.1186/s12985-020-01414-x. Virol J. 2020. PMID: 33032637 Free PMC article.
-
Cucumber mosaic virus coat protein modulates the accumulation of 2b protein and antiviral silencing that causes symptom recovery in planta.PLoS Pathog. 2017 Jul 20;13(7):e1006522. doi: 10.1371/journal.ppat.1006522. eCollection 2017 Jul. PLoS Pathog. 2017. PMID: 28727810 Free PMC article.
-
Replication of alfamo- and ilarviruses: role of the coat protein.Annu Rev Phytopathol. 2005;43:39-62. doi: 10.1146/annurev.phyto.43.101804.120505. Annu Rev Phytopathol. 2005. PMID: 16078876 Review.
-
The molecular biology of ilarviruses.Adv Virus Res. 2013;87:139-81. doi: 10.1016/B978-0-12-407698-3.00005-3. Adv Virus Res. 2013. PMID: 23809923 Review.
Cited by
-
Development and application of an infectious clone and gene silencing vector derived from blackberry chlorotic ringspot virus.Virus Res. 2024 Dec;350:199460. doi: 10.1016/j.virusres.2024.199460. Virus Res. 2024. PMID: 39233279 Free PMC article.
-
ICTV Virus Taxonomy Profile: Bromoviridae 2025.J Gen Virol. 2025 Jan;106(1):002069. doi: 10.1099/jgv.0.002069. J Gen Virol. 2025. PMID: 39888330 Free PMC article. Review.
References
-
- Brault V, Mutterer J, Scheidecker D, Simonis MT, Herrbach E, Richards K et al.. Effects of point mutations in the readthrough domain of the beet western yellows virus minor capsid protein on virus accumulation in planta and on transmission by aphids. J Virol. 2000;74: 1140–1148. doi: 10.1128/jvi.74.3.1140-1148.2000 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

