Photobiomodulation mitigates Bothrops jararacussu venom-induced damage in myoblast cells by enhancing myogenic factors and reducing cytokine production
- PMID: 38814992
- PMCID: PMC11192417
- DOI: 10.1371/journal.pntd.0012227
Photobiomodulation mitigates Bothrops jararacussu venom-induced damage in myoblast cells by enhancing myogenic factors and reducing cytokine production
Abstract
Background: Photobiomodulation has exhibited promise in mitigating the local effects induced by Bothrops snakebite envenoming; however, the mechanisms underlying this protection are not yet fully understood. Herein, the effectiveness of photobiomodulation effects on regenerative response of C2C12 myoblast cells following exposure to Bothrops jararacussu venom (BjsuV), as well as the mechanisms involved was investigated.
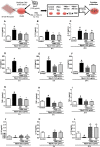
Methodology/principal findings: C2C12 myoblast cells were exposed to BjsuV (12.5 μg/mL) and irradiated once for 10 seconds with laser light of 660 nm (14.08 mW; 0.04 cm2; 352 mW/cm2) or 780 nm (17.6 mW; 0.04 cm2; 440 mW/ cm2) to provide energy densities of 3.52 and 4.4 J/cm2, and total energies of 0.1408 and 0.176 J, respectively. Cell migration was assessed through a wound-healing assay. The expression of MAPK p38-α, NF-Кβ, Myf5, Pax-7, MyoD, and myogenin proteins were assessed by western blotting analysis. In addition, interleukin IL1-β, IL-6, TNF-alfa and IL-10 levels were measured in the supernatant by ELISA. The PBM applied to C2C12 cells exposed to BjsuV promoted cell migration, increase the expression of myogenic factors (Pax7, MyF5, MyoD and myogenin), reduced the levels of proinflammatory cytokines, IL1-β, IL-6, TNF-alfa, and increased the levels of anti-inflammatory cytokine IL-10. In addition, PBM downregulates the expression of NF-kB, and had no effect on p38 MAKP.
Conclusion/significance: These data demonstrated that protection of the muscle cell by PBM seems to be related to the increase of myogenic factors as well as the modulation of inflammatory mediators. PBM therapy may offer a new therapeutic strategy to address the local effects of snakebite envenoming by promoting muscle regeneration and reducing the inflammatory process.
Copyright: © 2024 Silva et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

