Pharmacological modulation of septins restores calcium homeostasis and is neuroprotective in models of Alzheimer's disease
- PMID: 38815015
- PMCID: PMC11827694
- DOI: 10.1126/science.add6260
Pharmacological modulation of septins restores calcium homeostasis and is neuroprotective in models of Alzheimer's disease
Abstract
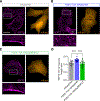
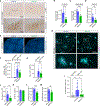
Abnormal calcium signaling is a central pathological component of Alzheimer's disease (AD). Here, we describe the identification of a class of compounds called ReS19-T, which are able to restore calcium homeostasis in cell-based models of tau pathology. Aberrant tau accumulation leads to uncontrolled activation of store-operated calcium channels (SOCCs) by remodeling septin filaments at the cell cortex. Binding of ReS19-T to septins restores filament assembly in the disease state and restrains calcium entry through SOCCs. In amyloid-β and tau-driven mouse models of disease, ReS19-T agents restored synaptic plasticity, normalized brain network activity, and attenuated the development of both amyloid-β and tau pathology. Our findings identify the septin cytoskeleton as a potential therapeutic target for the development of disease-modifying AD treatments.
Conflict of interest statement
H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). J.L.C. has provided consultation to Acadia, Actinogen, Acumen, AlphaCognition, ALZpath, Aprinoia, AriBio, Artery, Biogen, Biohaven, BioVie, BioXcel, Bristol-Myers Squib, Cassava, Cerecin, Diadem, Eisai, GAP Foundation, GemVax, Janssen, Jocasta, Karuna, Lighthouse, Lilly, Lundbeck, LSP/eqt, Merck, NervGen, New Amsterdam, Novo Nordisk, Oligomerix, Optoceutics, Ono, Otsuka, Oxford Brain Diagnostics, Prothena, reMYND, Roche, Sage Therapeutics, Signant Health, Simcere, Sinaptica, Suven, TrueBinding, Vaxxinity, and Wren pharmaceutical, assessment, and investment companies. J.W. and S.W. are cofounders and shareholders of reMYND. G.G. is a consultant for reMYND and owns reMYND warrants and shares. M.Fi. owns reMYND warrants. J.d.W. is cofounder of Augustine Tx and served as scientific advisory board member for Augustine Tx. J.T. is cofounder of Orionis Biosciences. K.Pr. and G.G. are inventors on patent WO2013/004642 held by reMYND NV: “1,2,4-thiadiazol-5-ylpiperazine derivatives useful in the treatment of neurodegenerative diseases.” K.Pr., M.V., M.Fi., and G.G. are inventors on patent application EP23209465.6 submitted by reMYND NV: “Modulators of septin 6 for use in the prevention and/or treatment of neurodegenerative disorders.”
Figures




Comment in
-
Rebalancing calcium in Alzheimer disease.Nat Rev Drug Discov. 2024 Aug;23(8):580. doi: 10.1038/d41573-024-00115-2. Nat Rev Drug Discov. 2024. PMID: 38969746 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

