Structural basis for inhibition of the lysosomal two-pore channel TPC2 by a small molecule antagonist
- PMID: 38815576
- PMCID: PMC11511679
- DOI: 10.1016/j.str.2024.05.005
Structural basis for inhibition of the lysosomal two-pore channel TPC2 by a small molecule antagonist
Abstract
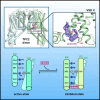
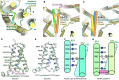
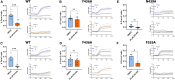
Two pore channels are lysosomal cation channels with crucial roles in tumor angiogenesis and viral release from endosomes. Inhibition of the two-pore channel 2 (TPC2) has emerged as potential therapeutic strategy for the treatment of cancers and viral infections, including Ebola and COVID-19. Here, we demonstrate that antagonist SG-094, a synthetic analog of the Chinese alkaloid medicine tetrandrine with increased potency and reduced toxicity, induces asymmetrical structural changes leading to a single binding pocket at only one intersubunit interface within the asymmetrical dimer. Supported by functional characterization of mutants by Ca2+ imaging and patch clamp experiments, we identify key residues in S1 and S4 involved in compound binding to the voltage sensing domain II. SG-094 arrests IIS4 in a downward shifted state which prevents pore opening via the IIS4/S5 linker, hence resembling gating modifiers of canonical VGICs. These findings may guide the rational development of new therapeutics antagonizing TPC2 activity.
Keywords: SG-094; TPC2; antagonist; cryo-EM; electrophysiology; ion channel; structural biology; two-pore channel; voltage-sensing domain.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




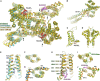
References
-
- Pitt S.J., Funnell T.M., Sitsapesan M., Venturi E., Rietdorf K., Ruas M., Ganesan A., Gosain R., Churchill G.C., Zhu M.X., et al. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+ J. Biol. Chem. 2010;285:35039–35046. doi: 10.1074/jbc.M110.156927. - DOI - PMC - PubMed
-
- García-Rúa V., Feijóo-Bandín S., Rodríguez-Penas D., Mosquera-Leal A., Abu-Assi E., Beiras A., María Seoane L., Lear P., Parrington J., Portolés M., et al. Endolysosomal two-pore channels regulate autophagy in cardiomyocytes. J. Physiol. 2016;594:3061–3077. doi: 10.1113/jp271332. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

