Convalescent human plasma candidate reference materials protect against Crimean-Congo haemorrhagic fever virus (CCHFV) challenge in an A129 mouse model
- PMID: 38815869
- PMCID: PMC11169527
- DOI: 10.1016/j.virusres.2024.199409
Convalescent human plasma candidate reference materials protect against Crimean-Congo haemorrhagic fever virus (CCHFV) challenge in an A129 mouse model
Abstract
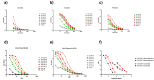
Crimean-Congo Haemorrhagic Fever Virus (CCHFV) is spread by infected ticks or direct contact with blood, tissues and fluids from infected patients or livestock. Infection with CCHFV causes severe haemorrhagic fever in humans which is fatal in up to 83 % of cases. CCHFV is listed as a priority pathogen by the World Health Organization (WHO) and there are currently no widely-approved vaccines. Defining a serological correlate of protection against CCHFV infection would support the development of vaccines by providing a 'target threshold' for pre-clinical and clinical immunogenicity studies to achieve in subjects and potentially obviate the need for in vivo protection studies. We therefore sought to establish titratable protection against CCHFV using pooled human convalescent plasma, in a mouse model. Convalescent plasma collected from seven individuals with a known previous CCHFV virus infection were characterised using binding antibody and neutralisation assays. All plasma recognised nucleoprotein and the Gc glycoprotein, but some had a lower Gn glycoprotein response by ELISA. Pooled plasma and two individual donations from convalescent donors were administered intraperitoneally to A129 mice 24 h prior to intradermal challenge with CCHFV (strain IbAr10200). A partial protective effect was observed with all three convalescent plasmas characterised by longer survival post-challenge and reduced clinical score. These protective responses were titratable. Further characterisation of the serological reactivities within these samples will establish their value as reference materials to support assay harmonisation and accelerate vaccine development for CCHFV.
Keywords: CCHF; CCHFV; Convalescent; Immunity; Infection; Mouse model; Vaccine.
Crown Copyright © 2024. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Shayan S., Bokaean M., Shahrivar M.R., Chinikar S. Crimean-Congo hemorrhagic fever. Lab. Med. 2015;46(3):180–189. - PubMed
-
- Bente D.A., Forrester N.L., Watts D.M., McAuley A.J., Whitehouse C.A., Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100(1):159–189. - PubMed
-
- Serretiello E., Astorri R., Chianese A., Stelitano D., Zannella C., Folliero V., et al. The emerging tick-borne Crimean-Congo haemorrhagic fever virus: a narrative review. Travel. Med. Infect. Dis. 2020;37 - PubMed
-
- Casals J., Tignor G.H. The Nairovirus genus: serological relationships. Intervirology. 1980;14(3–4):144–147. - PubMed
-
- Fillatre P., Revest M., Tattevin P. Crimean-Congo hemorrhagic fever: an update. Med. Mal. Infect. 2019;49(8):574–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

