A 3D Bioprinted Cortical Organoid Platform for Modeling Human Brain Development
- PMID: 38815975
- PMCID: PMC11518656
- DOI: 10.1002/adhm.202401603
A 3D Bioprinted Cortical Organoid Platform for Modeling Human Brain Development
Abstract
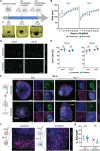
The ability to promote three-dimensional (3D) self-organization of induced pluripotent stem cells into complex tissue structures called organoids presents new opportunities for the field of developmental biology. Brain organoids have been used to investigate principles of neurodevelopment and neuropsychiatric disorders and serve as a drug screening and discovery platform. However, brain organoid cultures are currently limited by a lacking ability to precisely control their extracellular environment. Here, this work employs 3D bioprinting to generate a high-throughput, tunable, and reproducible scaffold for controlling organoid development and patterning. Additionally, this approach supports the coculture of organoids and vascular cells in a custom architecture containing interconnected endothelialized channels. Printing fidelity and mechanical assessments confirm that fabricated scaffolds closely match intended design features and exhibit stiffness values reflective of the developing human brain. Using organoid growth, viability, cytoarchitecture, proliferation, and transcriptomic benchmarks, this work finds that organoids cultured within the bioprinted scaffold long-term are healthy and have expected neuroectodermal differentiation. Lastly, this work confirms that the endothelial cells (ECs) in printed channel structures can migrate toward and infiltrate into the embedded organoids. This work demonstrates a tunable 3D culturing platform that can be used to create more complex and accurate models of human brain development and underlying diseases.
Keywords: 3D bioprinting; brain organoids; extracellular matrix; induced pluripotent stem cells; vasculature.
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Qian X., Nguyen H. N., Song M. M., Hadiono C., Ogden S. C., Hammack C., Yao B., Hamersky G. R., Jacob F., Zhong C., Yoon K. J., Jeang W., Lin L., Li Y., Thakor J., Berg D. A., Zhang C., Kang E., Chickering M., Nauen D., Ho C. Y., Wen Z., Christian K. M., Shi P. Y., Maher B. J., Wu H., Jin P., Tang H., Song H., Ming G. L., Cell 2016, 165, 1238. - PMC - PubMed
MeSH terms
Grants and funding
- R01 MH126195/MH/NIMH NIH HHS/United States
- UL1TR002378/National Center for Georgia Clinical & Translational Science Alliance of the National Institutes of Health
- ECCS-2025462/National Science Foundation
- R01MH126195/NH/NIH HHS/United States
- 1937971/National Science Foundation Graduate Research Fellowship Program
LinkOut - more resources
Full Text Sources

